8/10/2023
Condensing agents are widely used. With the development of pharmaceutical industry, polypeptide reagents, proteins and polymer materials, condensing agents are used more and more in organic synthesis, and the demand is also increasing year by year.
Condensing agents are widely used. With the development of the pharmaceutical industry, polypeptide reagents, proteins, and polymer materials, condensing agents are used more and more in organic synthesis, and the demand is also increasing year by year. To put it bluntly, condensation agents are the link that links acids and amines together. Commonly used condensation agents generally fall into the following categories:
1.1 Carbodiimide condensation agents: such as DIC, DCC, EDCI, etc.
1.2 Onium salt condensing agents: HATU, HBTU, HCTU, TBTU, HAPyU, BOP, PyBOP, etc.
1.3 Organophosphorus condensing agent: DPP-Cl, DPPA, MPTA, BOP-Cl, T 3 P, T 4 P, etc.
1.4 Other types of condensation agents: carbonyl imidazole CDI, preparation of mixed acid anhydride method, dioxime cyanide ethyl acetate derivatives, diphenylsilane, etc.
2.1 Carbodiimide condensing agents
The reaction mechanism of this type of condensing agent is the same, all acid and carbodiimide react to form an active intermediate, and then the amino group or hydroxyl nucleophilic attack forms a condensation product, while the carbodiimide condensing agent forms an active intermediate that is insoluble in organic solvents (but soluble in organic solvents). from alcohols) as a by-product of urea. The use of a carbodiimide condensing agent will cause the racemization of chiral compounds, so it cannot be used for the condensation of chiral compounds.
The use of this type of condensation agent generally requires the addition of an acylation catalyst or activator, such as 4-N, N-dimethylpyridine (DMAP), 1-hydroxybenzotriazole (HOBt), etc., which is mainly due to the reaction The addition intermediate of acid to carbodiimide in the first stage is not stable. If it is not converted into the corresponding active ester or active amide without an acylation catalyst, it will itself be rearranged into the corresponding stable by-product of urea, especially when using EDCI, it is often used in combination with HOBt. When we use carbodiimide condensation, the order of addition is generally that the acid and amine are added to the system first, and the condensing agent is added last. Most of the carbodiimide condensation reactions are Thermal reactions, so feeding is usually done under ice-water bath conditions.
Take DCC condensation as an example:
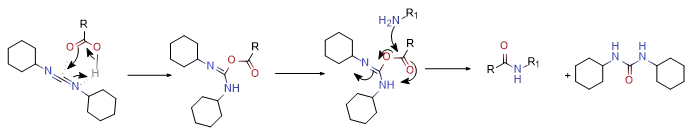
Usually, when this type of reaction generates an intermediate state with an acid, rearrangement of the intermediate state often occurs if a nucleophilic element (amine or hydroxyl) is not encountered.
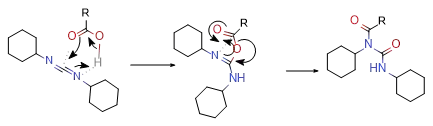
So often if the order of our addition is incorrect, the by-products we get will not only have the structure of urea, but you will also find other impurities produced by rearrangement.
2.2 Onium salt condensing agent
Onium salt condensation agents have high reactivity and are generally divided into two categories: carbonium salt condensation agents and phosphonium salt condensation agents. HATU, HBTU, HCTU, TBTU, etc. belong to the carbenium salt condensation agents. HAPyU, BOP, PyBOP, etc. are phosphonium salt condensing agents, and their reaction mechanisms are the same.
2.2.1 Carbonium salt condensing agents
Commonly used condensation agents of carbonium salts include HATU, HBTU, TBTU, etc. These condensation agents have higher activity and often do not need to add additional HOBt or HOAt, but their costs are also high. This type of condensation agent will produce by-products when the local concentration is uneven. Therefore, during the reaction, the general order of addition should be to add acid, alkali, and HATU to the solvent, stir evenly, and then add the amine. Avoid by-products. Taking HATU as an example, first, the acid forms a nucleophile under the action of a base and attacks the carbocation to form an intermediate state of acyloxy carbocation. Then this active intermediate is attacked by a benzotriazoloxy group to form active esters and amines. The nucleophile passes through an active ester, similar to the amine transesterification reaction, to generate the target product and by-product HOBt. Its mechanism is shown in the figure below.
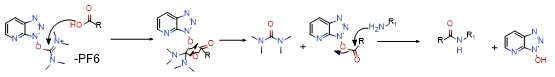
2.2.2 Condensing agent method of phosphonium salts
The main mechanism of action of this type of condensation agent is that under alkaline conditions, the carboxyl anion attacks the condensation reagent to generate the corresponding acyloxy phosphorus cation, and then the active intermediate is attacked by the benzotriazole group to form an active ester, and then reacts with the amino group The advantage of forming an amide bond is that the reaction conditions are mild and the condensation rate is fast. It can be applied to low-activity acid/amine condensation reactions. Taking PyBOP as an example, its mechanism of action is shown in the figure below (similar to carbon phosphonium salts).
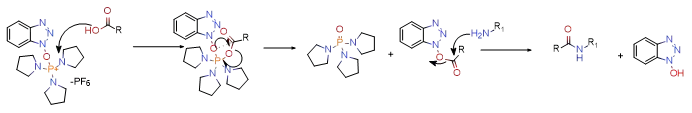
2.3 Organophosphorus condensation agent
Among these types of phosphate ester and phosphoramide condensation agents, the most commonly used are T 3 P and BOP-Cl. The reaction process mainly achieves condensation through the mixed anhydride intermediate state.
2.3.1 BOP-Cl is particularly suitable for the synthesis of amino acids. It has a high yield and is not easy to racemize. However, its disadvantage is that when the reactivity of the amine is low, acylated oxalidines are often obtained, but the solubility of BOP-Cl is relatively low. Poor, so the reaction time is longer and usually carried out in DMF, so the production application is affected to a certain extent. The reaction mechanism is as follows.
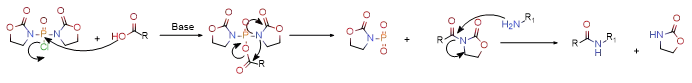
2.3.2T 3 P (n-propyl phosphoric anhydride) and T 4 P (n-butyl phosphoric anhydride) are dissolved in ethyl acetate, acetonitrile, or DMF. They have good solubility and convenient post-processing. They are often used in large-scale production. The by-products are easily removed by washing with water. For carboxylic acid substrates containing α-chiral centers, T 3 P and T 4 P can well inhibit epimerization. The reaction mechanism is shown below.
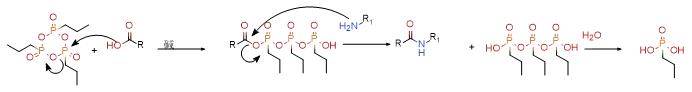
2.4 Other types of condensing agents
There are many other types of condensing agents: carbonyl imidazole CDI, preparation of mixed acid anhydride, dioxime ethyl cyanacetate derivatives, diphenylsilane, etc. I will not describe them one by one here but mainly introduce CDI.
CDI (carbonyldiimidazole) is usually used in THF and dichloromethane. Sometimes acetonitrile and DMF systems are used. However, the temperature needs to be controlled when used in DMF. When the temperature is high, the intermediate state generated by the reaction between acid and CDI will react with dichloromethane. Methylamine reacts to generate impurities, and the amount of CDI should not be too large, generally 1.1-1.2 equivalents. It is not advisable to add too much here, otherwise the subsequent amine will react with the unreacted CDI to generate impurities. The reaction process is that the acid first attacks the carbonyl carbon nucleophilically, releasing a molecule of imidazole to generate an unstable intermediate. The imidazole attacks the carbonyl carbon, generating an active carbonyl imidazole, and simultaneously releases carbon dioxide and imidazole. Finally, a nucleophilic amine is added to attack the carbonyl carbon, releasing imidazole to generate an amide. the process of. The feeding sequence is generally as follows: dissolve the acid into the solvent, add CDI (with a gas release process), stir for a period to generate an intermediate state, and then add the amine. For amines with low activity, you can choose to heat them. CDI easily absorbs moisture, so you need to pay attention to moisture-proof storage and use. Because the by-product imidazole produced by the reaction is easily soluble in water and easy to remove, it is also widely used in production. The reaction mechanism is shown below.

This article only gives a general explanation of commonly used condensation agents, and other condensation agents are not introduced. For more information, please stay tuned.
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.