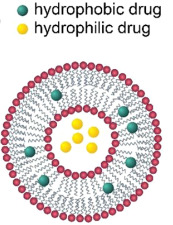
Liposomes are the precursor of lipid nanoparticles. They are small closed vesicles formed spontaneously from a phospholipid bilayer. They are similar in structure to cell membranes and can simulate the properties of cell membranes. Liposomes were discovered by Bangham et al. in the 1960s and were initially used as a simple model to study the structure and function of cell membranes. With the advancement of technology and more in-depth research, liposomes have gradually developed into a potential drug delivery carrier. Due to their amphipathic structure, liposomes can carry various drugs. Hydrophilic drugs can be enclosed in the aqueous interior region of liposomes, while hydrophobic drugs can be encapsulated in the hydrocarbon chain region of the lipid bilayer, as shown below.
Liposome composition
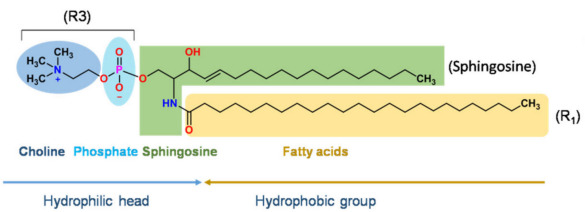
Liposomes used for drug delivery are mainly composed of various types of phospholipids and cholesterol, of which phospholipids (glycerophospholipids and sphingomyelin) are their basic skeleton. Glycerophospholipids are lipids with glycerol as the backbone, and sphingomyelin is a lipid with sphingosine as the backbone. The following figure shows the basic structures of glycerophospholipids and sphingomyelin.
Schematic diagram of the structure of glycerophospholipids
Schematic diagram of the structure of sphingomyelin
Note: R1 and R2 can be saturated fatty acids or unsaturated fatty acids, such as lauric acid, palmitic acid, oleic acid, erucic acid, etc. R3 can be neutral phosphatidylcholine (PC), phosphatidylethanolamine (PE), or charged phosphatidylserine (PS), phosphatidylinositol (PI), or phosphatidic acid at different pH values. (PA), Phosphatidylglycerol (PG)
Cholesterol is an amphipathic neutral lipid that can adjust the lipid bilayer's fluidity, increase liposome stability, reduce drug leakage, and play a role in prolonging and controlling drug release.
In addition to phospholipids and cholesterol, membrane materials such as polysaccharides (such as oligosaccharides, chitosan, and hyaluronic acid) and surfactants can also be added to liposomes to improve liposome stability and regulate drug release.
Liposome Classification
According to different structures and properties, liposomes can be divided into types such as unilamellar liposomes, multilamellar liposomes, and multivesicular liposomes. In addition, according to the charge carried by liposomes, they can be divided into cationic liposomes (such as DOTAP), anionic liposomes (such as DOPS), and neutral liposomes (such as DSPE). According to different functions, there are also long-circulation liposomes, glycosyl-modified liposomes, thermo-sensitive liposomes, pH-sensitive liposomes, immunoliposomes, magnetic liposomes, elastic liposomes, etc.
Liposome advantages and stability
Liposomes are thermally unstable systems and are prone to problems such as drug leakage and degradation. To achieve targeted therapy, stability is an indispensable factor for liposomes. The stability of liposomes includes physical stability, chemical stability, and biological stability. Physical and chemical stability generally refers to the ability of a liposome formulation to maintain its properties over a certain period. Phospholipids are prone to chemical degradation reactions, such as hydrolysis of ester bonds and peroxidation of unsaturated acyl chains, and these phenomena can affect the long-term stability of liposome formulations. In addition, to maintain the integrity of the liposome vesicle structure, it is necessary to balance the various interaction forces within and between liposomes. Choosing saturated phospholipids or low saturation phospholipids, appropriate bilayer concentration, appropriate buffer types, and adding antioxidants, metal chelators, and cryoprotectants can all increase the stability of liposomes.
Biological stability refers to the ability of liposomes to maintain their integrity in the presence of serum proteins. In the blood, after liposomes bind to serum proteins, opsonization occurs, resulting in rapid clearance of liposomes. To enhance biological stability, substances such as polyethylene glycol (PEG) can be added to avoid opsonization and prolong the circulation time of liposomes in the blood.
Liposomes are similar in structure to biological membranes and have good biocompatibility. As drug carriers, liposomes have the following advantages:
1. Can carry different types of drugs/genes: liposomes have a special bilayer structure that can seal and protect many different types of drugs and genes, including water-soluble and fat-soluble drugs.
2. Potential for drug administration through different routes: Liposomes can be administered through different routes, including oral administration, injection, topical application, etc. The most suitable route of administration can be selected according to specific needs.
3. Prevent chemical and biological degradation: Liposomes have a similar structure to cell membranes and can protect drugs from chemical and biological degradation, extend the half-life of drugs, and improve the stability and bioavailability of drugs.
4. Reduce non-specific side effects and toxicity of drugs: Liposomes can seal drugs inside, reduce the impact of drugs on non-target tissues, reduce non-specific side effects and toxicity, and improve the efficacy and therapeutic indicators of drugs.
5. Versatile chemical modification and targeting capabilities: Liposomes can be chemically modified to achieve targeting effects by attaching specific ligands or functional groups to improve drug targeting and selectivity.
6. Compatible with biodegradable and non-toxic materials: Liposomes can be compatible with biodegradable and non-toxic materials to reduce adverse effects on the human body.
Liposome Application
Because liposomes have good biocompatibility, nontoxicity, and diverse drug-carrying capabilities, liposomes have broad application prospects in drug delivery systems. The success of Doxil's research has inspired the research and development of liposome drug delivery systems, so liposomes are widely used in various fields of disease treatment.
The most important application of liposomes in drug delivery is cancer treatment. Cancer, a disease in which healthy cells of the body divide uncontrollably, is considered one of the major medical challenges of this century. Generally speaking, the vascular permeability of tumor sites is usually higher, and liposomes can be targeted into tumor tissues through capillary epithelial cells. In addition, the liposome structure is highly flexible and can be modified with various polymers and formulations. body, etc., to improve stability and achieve better-targeting effects. Liposomes are capable of carrying drugs with different physicochemical properties and are therefore considered ideal for drug delivery and cancer treatment in nanomedicine. In the field of cancer treatment, liposome drug delivery systems have shown great potential. Over the years, with the deepening of research, the therapeutic approaches and methods of liposomes as anti-tumor drug carriers have become more and more widespread. Currently, there are some drugs used for cancer treatment such as Marqibo® (vincristine), Lipusu ( Paclitaxel), and Onivyde® (irinotecan), etc.
Another important area of application is antifungal treatment. Fungal infections pose an increasing threat to human health, especially for immunocompromised populations, with invasive fungal infections causing particularly high morbidity and mortality. As a natural barrier for fungi, biofilm will affect the effect of antifungal drugs on bacteria, reduce drug uptake, and make it difficult for antifungal drugs to be effective. As a drug carrier, liposomes have good biocompatibility and can reduce the toxic and side effects of drugs. A structure similar to a biological membrane can fuse with the microbial cell plasma membrane and release high concentrations of drugs into the cell membrane or cytoplasm, achieving more efficient delivery and avoiding drug efflux. Drugs currently used for antifungal treatment include amphotericin B liposomes, such as Ambisome®, Abelcet®, and Amphotec®.
In addition to cancer treatment and antifungal treatment, liposomes also have a wide range of applications in other fields. For example, in the treatment of eye diseases, liposome drug delivery systems are used to treat eye infections. In addition, liposomes can also be used for anti-malarial treatment, preparing liposome vaccines (such as influenza vaccines), and overcoming biological barriers (such as blood-brain barrier, treating Alzheimer's disease), and have achieved good results.
However, large-scale production of liposomes has been challenging and costly, leading to a high positioning of liposomes in the market. Nonetheless, with advances in science and technology, expectations remain high for the continued development of liposomes and nanomedicine as drug delivery systems.
References:
[1] Pattni, BS; Chupin, VV; Torchilin VP New Developments in Liposomal Drug Delivery[J]. Chem. Rev. 2015, 115, 10938-10966.
[2] Yang Fuyu. Liposome in Biofilm research and pharmacological applications[J]. Progress in Biochemistry and Biophysics. 1977, 06, 36-40.
[3] Tenchov, R.; Bird, R.; Zhou, QQ; et al. Lipid Nanoparticles ─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement[J]. ACS Nano. 2021, 15, 16982-17015.
[4] Liu, P.; Chen, GL; Zhang, JC A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives[J]. Molecules. 2022, 27, 1372-1394. [5]
Liu Yang, Lu Wanliang, Zhang Qiang. Liposome and nanoparticle drug delivery systems Research progress of [J]. Journal of the Chinese Academy of Medical Sciences. 2006, 28, 583-589.
[6] Hussain, A.; Singh, S.; Sharma, D.; et al. Elastic liposomes as novel carriers: recent advances in drug delivery[J]. Int J Nanomedicine. 2017, 12, 5087-5108.
[7] Torchilin, VP Recent advances with liposomes as pharmaceutical carriers[J]. Nat Rev Drug Discov. 2005, 4, 145–160.
[8 ] Filipczak, N.; Pan, JY; Yalamarty, SSK; et al. Recent advancements in liposome technology[J]. Advanced Drug Delivery Reviews. 2020, 156, 4-22.
[9] Large, DE; Abdelmessih, RG; Fink, EA; et al. Liposome composition in drug delivery design, synthesis, characterization, and clinical application[J]. Advanced Drug Delivery Reviews. 2021, 176, 113851 [
10] Huang Ziyuan, Sun Yuqi, Hu Haiyang, Zhuang Rui, Xu Qi , Chen Dawei. Research techniques and methods on the stability of liposome formulations[J]. Acta Pharmaceutical Sinica, 2016, 51, 356-361.
[11] Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review[J]. Current Drug Delivery. 2007, 4, 297-305.
[12] Ebrahim, S.; Peyman, GA; Lee, PJ Applications of Liposomes in Ophthalmology[J]. Surv Ophthalmol. 2005, 50, 167-182.
[13] Nsairat, H.; Khater, D.; Sayed, U.; et al. Liposomes: structure, composition, types, and clinical applications[J]. Heliyon. 2022, 8, 9394.
[14] Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy[J]. Nanoscale Res Lett. 2021, 16, 95.
[15] Chen Shuisheng, Zhou Keqian, Li Xiaodong, Lu Quanzhen, Yu Yuan. Application and mechanism of nano drug delivery systems in the treatment of antifungal infections. Acta Pharmaceutical Sinica, 2021, 56, 1893-1901.