About one-third of the 200 best-selling drugs in the world in 2019 contain amide bonds [2,3]. Developing efficient amide synthesis methods has long been one of the main goals of synthetic chemistry. The green and environmentally friendly amide synthesis method has been identified as a key research area by the American Chemical Society's Green Chemistry Institute and is being continuously developed [4].
At the same time, the formation of amide bonds is one of the most basic and core reactions in the field of peptide and protein synthesis. After long-term research, scientists have developed many methods for constructing amide bonds, such as mixed anhydride, acyl azide method, condensation agent method and other methods, but the most widely used is still the condensation agent-mediated amide synthesis method. Traditional condensation agents still have some defects in reaction efficiency, optical purity, steric hindrance, stability, separation, etc. In addition, the low atomic economy of solid-phase peptide synthesis has brought great challenges to sustainable development. Therefore, developing an efficient, simple, and optically pure condensation reagent is a major problem that plagues researchers.
In recent years, Professor Zhao Junfeng's team has been committed to solving the difficult problems in the field of peptide and protein chemical synthesis by developing new reagents and new reactions around the formation of amide bonds. Through unremitting efforts, they developed a new structural alkynamide condensation reagent, Ynamide, which achieves efficient and racemization-free construction of amide bonds[5].
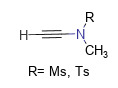
Representative products of this type of reagent are N-methylethynyl methanesulfonamide (MYMsA) and N-methylethynyl p-toluenesulfonamide (MYTsA). They have small molecular weights, do not require the addition of additional catalysts, are stable to air and water, and can almost quantitatively obtain the target amide under near-neutral conditions. More importantly, carboxylic acids containing α-chiral centers will not undergo racemization during the condensation process. Figure 1 shows the general structural formula of acetylene amide reagents [6].
Figure 1
The alkyne amide reagents developed by Professor Zhao's team are widely used and can be used in the synthesis of common amides and peptide fragments, esters and macrolides, thioamides, etc. The following will introduce in detail the research progress of this type of alkyne amide condensation reagents in synthetic applications.
1. Used for amide and peptide synthesis
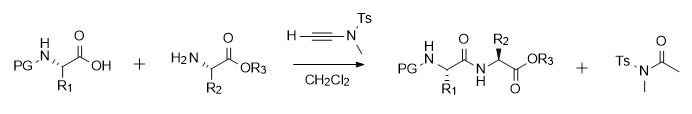
Alkynamide-mediated amide bond synthesis can proceed spontaneously and efficiently in both carboxylic acid activation and aminolysis reactions. Therefore, Professor Zhao's research group adopted a two-step one-pot method, that is, the activated intermediate was not separated and the subsequent reaction with amine was directly carried out, which simplified the operation process. This scheme has good universality. It is applicable to substrates with large steric hindrance and chiral amino acids without racemization. In addition, the alkynamide-mediated reaction is not only suitable for the synthesis of amides and dipeptides, but also can be used for the synthesis of peptide fragments (such as the synthesis of Leu-enkephalin with protective groups). In addition, this type of condensation reagent has good tolerance to many functional groups and can occur in the presence of amino acid side chain functional groups such as OH, SH, CONH2, and NH of indole [6].
Figure 2 Amide synthesis
2. Used for the synthesis of esters (thioesters) and macrolides
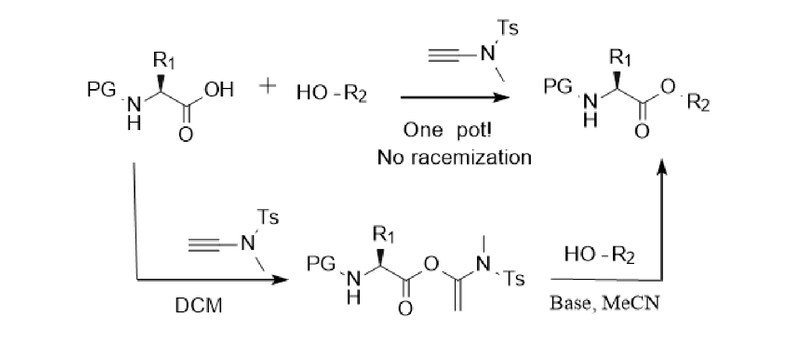
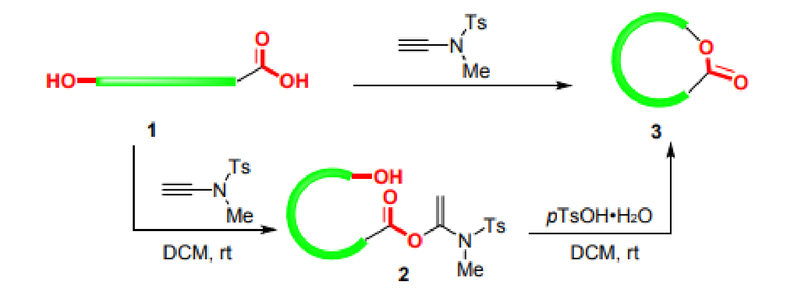
Ester bonds are important functional groups in many natural products and fine chemical products. Thiocarbonyl esters are also important intermediates for functional group transformation. Macrolides are the core skeletons of many drugs and natural products. Since the activated esters produced by the reaction of alkyne amide reagents with carboxylic acids are stable and can be stored in a refrigerator for up to half a year, Professor Zhao's research group further expanded the application of alkyne amide reagents and developed intermolecular esterification and macrolide synthesis schemes, as shown in Figure 3: Under alkaline conditions, acetonitrile can be used as a solvent to smoothly achieve esterification reactions. For chiral α-amino acids, racemization can also be avoided under the catalysis of DIEA. The substrates are universal and can be used for (thiol) alcohols and (thio) phenols. In addition, the synthesis of macrolides has problems such as racemization, cis-trans isomerization, intermolecular polymerization and ring-closure side reactions, which have always troubled many researchers. Professor Zhao's research group successfully synthesized macrolides using alkyne amide reagents (Figure 4). Under the catalysis of p-toluenesulfonic acid hydrate, the two-step one-pot method was still used. Macrolides can be successfully synthesized at room temperature even at high concentrations, effectively avoiding problems such as racemization and cis-trans isomerization [7,8,9].
![highfine highfine]()
Figure 3 Intermolecular esterification
Figure 4 Synthesis of macrolides
3. Thioamides and thiopeptides
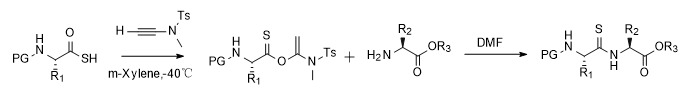
Precise modification and functionalization of peptides and proteins have become indispensable tools in chemical biology. Thioamide bonds can replace traditional peptide bonds to obtain different physical and chemical properties, such as enhancing the resistance of peptides to enzymatic degradation and making probes with unique spectral characteristics. Due to the limitations of synthetic methods, the application of thioamides in the field of protein biology is also limited. In response to the challenge of introducing thioamide bonds into proteins and peptides, Professor Zhao Junfeng's research group developed a thioamide synthesis scheme mediated by alkyne amide. In dichloromethane or DMF, thioamides can be obtained without racemization. In solid-phase peptide synthesis, the activated ester intermediates prepared by thiocarboxylic acids and alkyne amides can be efficiently converted into target products (all 19 natural amino acids except histidine), thus laying the foundation for the study of thioamides in the chemical biology of peptides and proteins [10,11].
![highfine highfine]()
Figure 5 Synthesis of thioamide
Alkynamide condensation agents not only have the advantages of traditional condensation reagents and activated esters, but also make up for their shortcomings. They provide new methods for the synthesis of important substances such as amides and polypeptides, esters and macrolides, and thiopeptides, and have important scientific significance in the field of polypeptide and protein synthesis. In addition, through the efforts of our scientific researchers, Suzhou Haofan has been able to provide alkynamide condensation reagents. At the same time, our company is committed to the research and development and production of amide and polypeptide synthesis reagents. After 20 years of development and accumulation, our company has become the world's largest and most comprehensive supplier of amide synthesis reagents. The first to fourth generation condensation reagents are all on sale. Friends with needs are welcome to call us for inquiries.
references:
[1] Boström, J.; Brown, D.G.; Young, R.J.; et al. Expanding the medicinal chemistry synthetic toolbox[J]. Nat. Rev. Drug. Discov. 2018, 17, 709-727.
[2] Top 200 Drugs 2019——The Poster. https://www. pharmaexcipients.com/news/top-200-drugs-2019/ (accessed 2021-12-18)
[3] Magano, J. Large-Scale Amidations in Process Chemistry: Practical Considerations for Reagent Selection and Reaction Execution[J]. Org. Process Res. Dev. 2022, 26, 1562-1689.
[4] Bryan, M. C.; Dunn, P. J.; Entwistle, D.; et al. Key Green Chemistry research areas from a pharmaceutical manufacturers’ perspective revisited[J]. Green Chem. 2018, 20, 5082− 5103.
[5] Liu, T.; Xu, S.L.; Zhao, J.F. Recent Advances in Ynamide Coupling Reagent[J]. Chin. J. Org. Chem. 2021, 41, 873-887.
[6] Hu, L.; Xu, S.L.; Zhao, J.F.; et al. Ynamides as racemization-free coupling reagents for amide and peptide synthesis[J]. J. Am. Chem. Soc. 2016, 138, 13135-13138.
[7] Wang, X.W.; Yang, Y.; Zhao, J.F.; et al. Ynamide-Mediated Intermolecular Esterification[J]. J. Org. Chem. 2020, 85, 6188-6194.
[8] Yao, C.C.; Yang, J.H.; Zhao, J.F.; et al. Ynamide-Mediated Thionoester and Dithioester Syntheses[J]. Org. Lett. 2020, 22, 6628-6631.
[9] Yang, M.; Wang, X.W.; Zhao, J.F. Ynamide-Mediated Macrolactonization[J]. ACS Catal. 2020, 10, 5230-5235.
[10] Yang, J.H.; Wang, C.L.; Xu, S.L.; Zhao, J.F. Ynamide-Mediated Thiopeptide Synthesis[J]. Angew. Chem. Int. Ed. 2018, 58, 1382-1386.
[11] Yang, J.H.; Wang, C.C.; Zhao, J.F.; et al. Site-Specific Incorporation of Multiple Thioamide Substitutions into a Peptide Backbone via Solid Phase Peptide Synthesis[J]. J. Org. Chem. 2020, 85, 1484-1494.