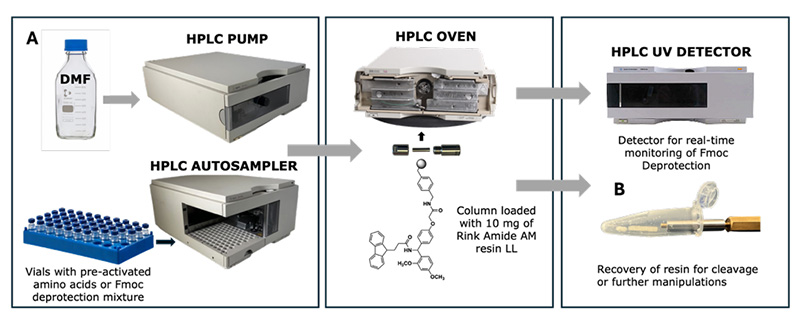
I. Background Introduction:
In recent years, the introduction of microwave-assisted synthesis and flow synthesis techniques has further improved the efficiency and yield of solid-phase synthesis. However, these techniques typically rely on expensive and highly specialized equipment, limiting their widespread adoption in routine laboratories. Furthermore, HATU, the activating reagent widely used in Fmoc solid-phase synthesis, suffers from poor thermal stability and safety concerns.
In light of this, Nicolas Winssinger's team at the University of Geneva, Switzerland, utilized a general-purpose liquid chromatography system, modifying it for flow chemistry techniques to achieve the transformation from a high-performance liquid chromatograph (HPLC) to a peptide synthesizer.
II. Liquid phase method:
HPLC is a commonly used separation and analysis technique. Samples are separated into components using a chromatographic column to determine sample purity. This team innovatively used a guard column pre-column filled with 10 mg of resin as a micro-flow reactor, with an internal 2 μm melt to prevent resin loss. Simultaneously, a DAD detector (detecting Fmoc deprotection byproducts) was used to provide real-time feedback on the synthesis results.
This scheme uses TBEC (1-tert-butyl-3-ethylcarbodiimide)/Oxyma (ethyl 2-oxime cyanoacetate) as the activation system, eliminating the need for additional alkali addition and pre-activation steps, simplifying the process and making it safer and more environmentally friendly.
Based on the above equipment and activation system, to explore synthesis conditions, phenylalanine pentamer was used as a model, and the conditions were optimized on the modified HPLC system. Experimental results showed that the optimized conditions successfully synthesized the target pentapeptide, achieving a crude product purity >93% and a single-step purity >99%.
The specific method is as follows:
| Instrument Model |
Agilent 1260 Infinity |
| Automatic sampler volume |
100 μL |
Column temperature |
80℃ |
| chromatographic column |
C18 silica gel column / Hypersil Gold C18 chromatography column |
Protective pillar |
Resin-filled (20 x 2 mm) |
| detector |
DAD detector |
Detection wavelength |
360 nm |
| Flow rate |
1.0 mL/min |
mobile phase |
Mobile phase A: Activated ester solution |
| Washing process |
Time/min |
0 |
0.1 |
1.5 |
2.0 |
| Mobile phase A |
100 |
100 |
100 |
100 |
III. Operating Procedures:
The entire synthesis process includes the following stages:
1. Weighing and loading resin: Weigh 10 mg of resin (such as Rink Amide resin) and fill it into the empty protective column, then seal and fix it.
2. Resin swelling: Immerse the resin-loaded protective column in dichloromethane and allow it to swell for 3-5 minutes.
3. Installation: Set the column oven temperature to 80℃ and install the protective column into the corresponding holder. 4.
Reagent solution preparation:
Sample solution: Prepare 0.6 M Fmoc amino acid, TBEC, and Oxyma NMP solution, and mix them in equal volumes to obtain a 0.2 M solution.
Deprotection solution: Add 20% piperidine to DMF and transfer it to an autosampler vial for later use.
5. Programming the injection sequence: Set the instrument to alternately inject the activated amino acid derivative solution (for coupling) and the 20% piperidine DMF solution (for deprotection). In addition, deprotection should be performed twice consecutively before the start of synthesis to ensure complete removal of the initial Fmoc group.
6. Resin lysis and deprotection: Immerse the guard column in dichloromethane to remove residual DMF; then remove the resin and immerse it in a TFA solution (TFA/triisopropylsilane TIS/H2O = 95:2.5:2.5) for 3 hours to achieve complete dissociation and deprotection. For specific sequences containing cysteine, methionine, etc., a mixed lysis buffer of TFA/1,2-ethylenedithiol EDT/TIS/H2O = 90:5:2.5:2.5 can be used.
7. Product processing: Separate the resin to precipitate the crude peptide for further purification.
IV. Influencing Factors:
For racemic amino acids such as histidine and some special amino acids, the pre-activation time has a significant impact on their coupling efficiency. In the synthesis of 20 peptides, the team compared the coupling efficiency of freshly prepared active esters with those pre-activated for 24 hours, finding that Fmoc-Arg(pbf)-OH and Fmoc-His(Trt)-OH exhibited relatively low coupling efficiency after prolonged activation.
To further investigate the effect of pre-activation time on coupling efficiency, FRF tripeptides were synthesized using Fmoc-Arg(pbf)-OH as a raw material, with pre-activation times of 15 minutes and 24 hours, respectively. The results showed that the coupling efficiency significantly decreased after 24 hours of pre-activation, attributed to the intramolecular cyclization of Fmoc-Arg(pbf)-OH after activation, which reduces its reactivity. Repeated experiments verified that the ideal activation solution should be used within 4 hours of preparation.
V. Differential Isomerization:
Isomerization during peptide synthesis can affect stereochemical purity and alter biological activity. To assess the degree of racemization under these synthetic conditions, the team employed a liquid-phase synthesis method to investigate easily racemable amino acids such as proline, cysteine, and serine, and no racemization was observed in any of them. Further comparing the coupling effects of the TBEC/Oxyma system and the HATU/DIPEA system, the team used freshly activated His (Trt) for the reaction. The experiments revealed that the former exhibited epimerization of 3.6%, while the latter showed an isomerization rate of 4.8%.
VI. Practical Applications:
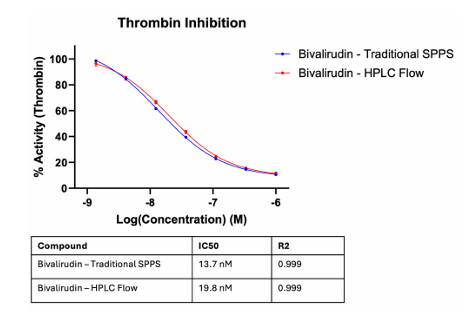
The team successfully synthesized peptides such as GLP-1 (30 amino acids) and bivalirudin (20 amino acids), with a yield of 52.1% for GLP-1 synthesized using a synthesis column containing 10 mg of resin. Regarding bioactivity, the crude bivalirudin synthesized by HPLC and the pure bivalirudin synthesized by conventional SPPS exhibited similar bioactivity (see figure below).
Figure 5
VII. Advantages and limitations:
The readily available HPLC system, applied to automated fluid synthesis of peptides, has the following advantages and disadvantages:
1. Advantages:
- Transform standard HPLC into a multifunctional synthetic platform;
- Employ a safer TBEC/Oxyma activation system;
- It can obtain high-purity peptides and monitor the reaction in real time;
- It exhibits good stereoselectivity and can synthesize polypeptides with complex sequences;
- It is economical, uses widely available HPLC equipment, reduces equipment costs, and can benefit more laboratories;
- It offers high flexibility and can be used for the synthesis of peptides/oligonucleotides. It also allows switching between synthesis and purification modes by changing the column.
2. Limitations:
- It cannot synthesize certain complex sequences;
- The reaction rates differ between different amino acids or peptide chains, requiring further optimization.
- The synthesis volume is small, and it is only suitable for synthesis at the microgram to milligram level.
VIII. Overall Conclusion:
This study provides a simple, safe, and economical peptide synthesis platform that enables simultaneous reaction and detection using a conventional HPLC system. This approach not only maintains the stereochemical integrity of the product but also allows for the synthesis of complex, biologically active sequence fragments, contributing to the wider adoption of flow synthesis techniques in more laboratories.
About Highfine Biotech:
Suzhou Highfine Biotech Co., Ltd. (Stock Code: 301393.SZ), founded in 2003 and headquartered in Suzhou High-tech Zone, is a national high-tech enterprise providing specialty raw materials to global pharmaceutical R&D and manufacturing companies. Its products are mainly used in peptide, nucleotide, and pharmaceutical synthesis, encompassing a wide range of products including condensing agents for specialty amide bond formation, protective agents, linkers, protein cross-linking agents for antibody-drug conjugates, molecular building blocks, liposomes, and phosphorus reagents. Currently, it has developed and produced over 1500 products.
After 22 years of unremitting efforts and accumulation, Highfine Biotech has continuously deepened its expertise in the global peptide synthesis reagent field and has now developed into a leading company with extensive customized product coverage and significant advantages in large-scale production, capable of meeting the specific needs of various customers. We sincerely invite customers interested in our products to contact us for further information and to discuss cooperation opportunities.
References:
[1] Romanens, P.; Barluenga, MD; Winssinger, N., et al. Peptides on Tap: Automated Flow Synthesis with Standard HPLC [J]. ChemRxiv. 2025.