Background information:
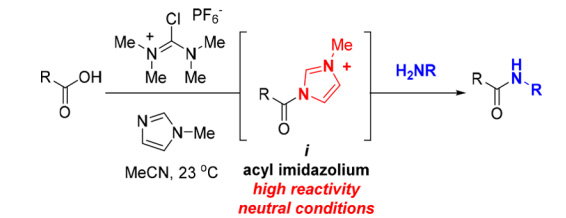
Among them, the TCFH-NMI combination is a representative of "old tree with new branches". This combination was originally used for the amidation reaction of carboxylic acids containing chiral centers and inactive amines. By generating highly reactive N-acylimidazolium salts in situ during the reaction, the reaction is driven (see Figure 1 below). It not only has excellent stereoselectivity, but also achieves kilogram-scale production (see the golden combination of efficient acid-amine condensation: TCFH-NMI).
Figure 1
A study by Takeda Corporation showed that the TCFH-NMI combination can yield amide products in high yields in aqueous solvents, revealing its application potential in aqueous solvents. Based on this, Bristol-Myers Squibb conducted further research: exploring the applicability and conditions of TCFH-NMI amidation under water as the primary reaction medium.
I. Reaction conditions:
The company explored and established a general operating procedure for the application of the TCFH-NMI combination in aqueous media: acid (500 mg, 1.0 eq) is placed in a 20 mL reaction flask, and aqueous solvent (water: 85%, organic solvent: tetrahydrofuran/acetonitrile/acetone, 15%), amine (1.1-1.3 eq), and NMI (3.5 eq) are added in sequence. Finally, TCFH (3*0.5 eq) is added in three batches with a 10-minute interval between batches, and the reaction is carried out for 30 min after the addition is completed.
1. Influence of solvent:
(1) Influence of water content: The reaction is usually carried out at a water content of 85%. For some compounds, when the water content is ≤50%, the reaction conversion rate decreases with the increase of water content; while when the water content exceeds 85%, the reaction conversion rate is comparable to that when pure acetonitrile is used as solvent .
(2) Selection of organic solvent: Acetone, tetrahydrofuran and acetonitrile can be substituted for each other. Among them, acetone is better as a co-solvent. Other water-soluble organic reagents can also be tried, accounting for 15%.
2. Amount of base:
NMI equivalent (2.1 and 3.5 eq) has no significant effect on conversion rate and epimerization.
II. Reaction substrate:
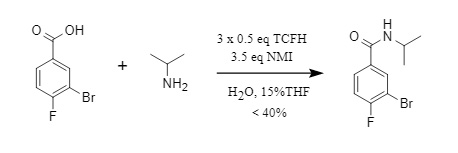
Richard J. Fox and Dung L. Golden et al. attempted to synthesize an intermediate for an oncology drug using the method published by Takeda Pharmaceutical (see Figure 2 below), but the conversion rate was less than 40%. Even changing the organic solvent and adding a surfactant did not significantly improve the conversion rate. This was presumably due to the steric hindrance effect of isopropylamine. After switching to an amine with less steric hindrance (such as an aniline derivative), the conversion rate was significantly improved.
Figure 2
Therefore, the team conducted further systematic experiments on various amine compounds and carboxylic acids and summarized the following rules:
1. When amine compounds
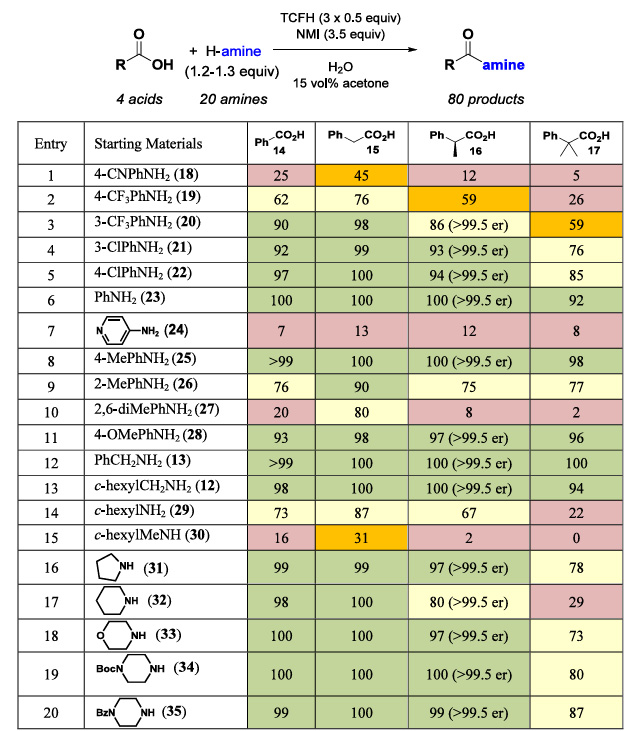
undergo condensation reactions in aqueous solution using TCFH-NMI, the greater the substrate steric hindrance, the lower the reaction conversion rate. Through experiments on amidation of 20 amine compounds with 4 carboxylic acids (see Figure 3 below), the following rules were obtained:
(1) Aromatic amines: The conversion rate is high when containing weak electron-withdrawing groups and electron-donating groups; the conversion rate is low when containing strong electron-withdrawing groups;
(2) Aliphatic amines: Suitable for strong nucleophilic amines.
Figure 3
Furthermore, when ammonia is used as the reaction substrate, the reaction is less effective under aqueous conditions (85% water), possibly because ammonia forms hydrogen bonds in water, reducing its nucleophilicity. The conversion rate significantly improves when the proportions of acetonitrile and water are roughly equal.
2. Carboxylic Acids
Besides steric hindrance, which, like amines, negatively impacts the reaction, the pKa of carboxylic acids and the electronic effects of substituents are also key factors influencing the reaction results.
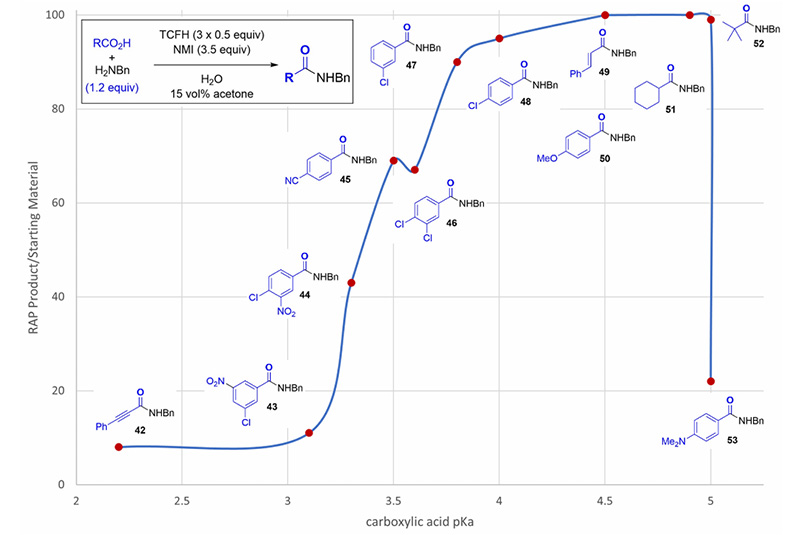
(1) pKa
Within the pKa range of 3.0-5.0, the larger the pKa value, the better the reaction effect. In the range of 3.8-5.0, the conversion rate can reach over 90% (see Figure 4).
Figure 4
(2) Electronic effects:
Hammett-style studies were conducted on the reaction of 23 different carboxylic acids with benzylamine. The results showed that
the conversion rate was the highest (>80%) when the substituents were medium-electron substituents (including electron-withdrawing and electron-donating) and neutral substituents; the conversion rate was slightly lower (60-80%) when the substituents were strong electron-withdrawing substituents; and the conversion rate was very poor (<40%) when the substituents were strong electron-donating substituents.
III. Differential Isomerization:
An excellent condensation reagent or reaction system must simultaneously achieve high reaction efficiency and excellent stereoselectivity. This team conducted epimerization experiments with benzylamine and various amino acids and peptides, achieving excellent conversion rates and enantiomeric ratios (er) values >99.9:0.1 for both N-Boc-phenylalanine and N-Boc-phenylglycine. However, when the dipeptide was tested with different amines, the er values were poor, which was speculated to be because the dipeptide formed a racemic oxazolone intermediate during the reaction.
IV. Practical Applications:
1. Gram-scale:
The team not only conducted extensive experiments screening reaction substrates but also scaled up the reaction at the gram scale. In an acetone/water ratio of 15%/85%, they reacted five different groups of carboxylic acids and amines, obtaining products with high purity and process quality intensity (PMI) far below the industry average, demonstrating high production efficiency and excellent production potential.
2. Synthesis of Active Molecules:
To further verify the application value of this reagent in aqueous systems, the team applied this system to the synthesis of active molecules such as Linrodostat, olaparib, rivaroxaban analogs, nilotinib, and imatinib. Except for nilotinib, which showed poor results due to poor substrate solubility and system viscosity, the target compounds were obtained with excellent yields and purity for the others.
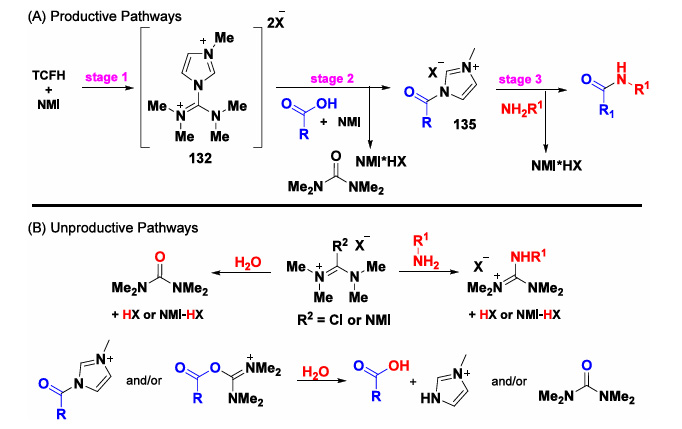
V. Reaction Mechanism:
The article begins by mentioning that the mechanism of action of TCFH-NMI is to promote the reaction by forming an active N-acylimidazolium salt. In aqueous systems, the reaction efficiency of this combination depends on the balance between the reaction pathway, i.e., the formation of the N-acylimidazolium salt, and the non-reactive hydrolysis pathway (see Figure 5 below). When carboxylic acids contain strong electron-withdrawing or strong electron-donating groups, amine compounds contain very weak electron-donating groups, and both have significant steric hindrance, the rate of the reaction pathway will be reduced, thereby increasing the proportion of the non-reactive pathway and reducing the yield of the product.
Figure 5
VI. Machine Learning Models:
The team also developed a supervised machine learning model to predict the conversion rate of the TCFH-NMI combination in an aqueous system for amidation, including the stereo and electronic properties of the carboxylic acid and amine. The model's predictive performance was evaluated using cross-validation and independent validation sets. The results showed that the model performed well in predicting the amidation conversion rate of this combination.
Overall conclusion:
In summary, the team studied over 100 cases of TCFH-NMI amidation using water as the primary reaction solvent. The combined data indicate that this combination is feasible in aqueous systems and, in many cases, allows for efficient and direct product separation. However, this reaction system is not a solution applicable to all substrate combinations. Nevertheless, this work provides valuable insights and inspiration for advancing the application of aqueous condensation reactions and other organic reactions in aqueous media.
About Highfine Biotech:
Suzhou Highfine Biotech Co., Ltd. (Stock Code: 301393.SZ), founded in 2003 and headquartered in Suzhou High-tech Zone, is a national high-tech enterprise providing specialty raw materials to global pharmaceutical R&D and manufacturing companies. Its products are mainly used in peptide, nucleotide, and pharmaceutical synthesis, encompassing a wide range of products including condensing agents for specialty amide bond formation, protective agents, linkers, protein cross-linking agents for antibody-drug conjugates, molecular building blocks, liposomes, and phosphorus reagents. Currently, it has developed and produced over 1500 products.
After 22 years of unremitting efforts and accumulation, Highfine Biotech has continuously deepened its expertise in the global peptide synthesis reagent field and has now developed into a leading company with extensive customized product coverage and significant advantages in large-scale production, capable of meeting the specific needs of various customers. We sincerely invite customers interested in our products to contact us for further information and to discuss cooperation opportunities.
References:
[1] Fox, RJ; Golden, DL; Chartrand, CC, et al. Tetramethylchloroformamidinium Hexafluorophosphate−N‑Methylimidazole Amidation in Water: Successes, Limitations, and a Regression Model for Prediction [J]. Org. Process Res. Dev., 2025.