This article will briefly introduce the phthaloyl series of amino protecting groups.
1. Introduction
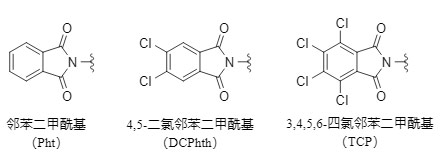
Phthaloyl and its derivatives are a type of commonly used amino protecting groups (see the structure in the figure below, collectively referred to as phthaloyl below, and specific protecting groups are represented by their abbreviations), which are widely used in the synthesis of complex organic molecules, especially peptides and sugars.
Figure 1 Common phthalic acid protecting groups
Phthaloyl is usually used to protect primary amines. It can remain stable under conventional acidic conditions, catalytic hydrogenolysis, and NH3(l)/Na reduction systems. Compared with other acyl protecting groups, phthaloyl can achieve full substitution of amino groups, thereby effectively inhibiting racemization in peptide synthesis. However, it is unstable under alkaline conditions and in the presence of strong nucleophiles, and hydrazine hydrate is often used for deprotection.
2. Introduction Method
2.1 Phthalic anhydride method
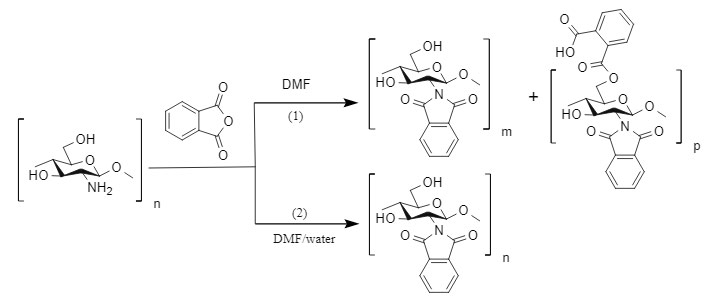
Kurita et al. used phthalic anhydride in a DMF solution containing 5% water at 120°C to introduce the Pht protecting group when protecting the amino group of chitosan, with a yield of >80%. Under this condition, the amino group can be selectively protected while the hydroxyl group is not affected (route 2). Kurita et al. also found that if the reaction is carried out in pure DMF, a by-product in which both the amino group and the hydroxyl group are protected will be obtained (route 1).
This method usually needs to be carried out at a higher temperature. If the molecule contains multiple amino groups (secondary amines and primary amines coexist), side reactions involving secondary amines are likely to occur. However, this method is relatively simple to post-process and is suitable for industrial production.
2.2 Monoethyl phthalate method
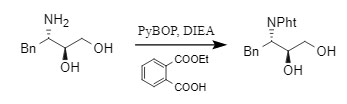
Aguilar et al. used monoethyl phthalate in the presence of a base (such as DIEA) and a condensation reagent PyBOP to promote the amide reaction, thereby introducing a Pht protecting group.
This method is relatively cumbersome and takes a long time to react. It is also not suitable for the coexistence of multiple amino groups.
2.3 N-ethoxycarbonylphthalimide method
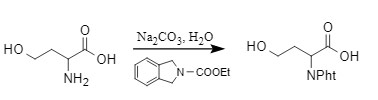
Jonghe et al. used N-ethoxycarbonylphthalimide to introduce the Pht protecting group under alkaline conditions when protecting the amino group.
This method is mild, low-cost, and can selectively protect primary amines in the presence of secondary amines. It is currently a more mainstream introduction method.
Khalifa et al. developed a method for efficiently introducing a Pht protecting group through N-benzenesulfonylphthalimide (prepared by the reaction of phthalimide and benzenesulfonyl chloride). This method can introduce Pht under the condition of acetonitrile reflux, with high yield and no need for additional base, and applies to a variety of aromatic amines and aliphatic amines.
3. Removal method
It is crucial to choose a suitable removal method, which not only has high removal efficiency, but also ensures that other protecting groups are not affected.
3.1 Hydrazinolysis method: Usually, the Pht protecting group is removed by hydrazine hydrolysis. Generally, it can be completely removed by refluxing in an alcohol solution of hydrazine hydrate. Under this condition, Cbz, Boc, Ts, Trt, and other protecting groups are not affected.
3.2 NaBH4/i-PrOH/AcOH method: This method is a supplement to the hydrazine hydrolysis method. When the hydrazine hydrolysis effect is not good, this condition can be used to remove Pht well.
3.3 Ethylene diamine method: The tetrachlorophthaloyl group (TCP) in this series is selectively removed in the presence of ethylenediamine, while the ester group and Pht are not affected.
4. Synthesis Strategy
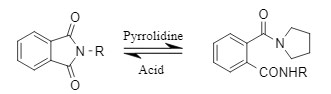
The Pht protecting group can be temporarily inactivated to ensure the normal progress of other reactions, and then the strategy of "resurrection" can be used in specific scenarios. Given the poor stability of Pht in the presence of alkaline and nucleophilic reagents, it can be reacted with tetrahydropyrrole to obtain a more stable derivative. After other reactions are completed, it can be regenerated into a Pht group under acidic conditions and continue to participate in subsequent reactions.
This article outlines the introduction and removal methods of the amino protecting group - phthaloyl and its expanded application in synthetic strategies, hoping to provide a reference and ideas for related workers.
References:
[1] Guo Yanhao, Hao Qinghui, Hao Siyuan, et al. Protection and deprotection of amino groups in chemical products[J]. Coal and Chemical Industry, 2022, 45, 106-112.
[2] Kurita, K.; lkeda, H.; Harata, M.; et al. Chemoselective Protection of the Amino Groups of Chitosan by Controlled Phthaloylation: Facile Preparation of a Precursor Useful for Chemical Modifications[J]. Biomacromolecules, 2002, 3.
[3] Aguilar, N.; Moyano, A.; Pericàs, MA; Riera, A. A General, Catalytic, and Enantioselective Synthesis of (S)-γ-[(S)-1-Aminoalkyl]-γ-lactones[J]. J. Org. Chem. 1998, 63, 3560-3567.
[4] Jonghe, SD; Lamote, I.; Herdewijn. P; et, al. Synthesis of Homoceramides, Novel Ceramide Analogues, and Their Lack of Effect on the Growth of Hippocampal Neurons[J]. J. Org. Chem. 2002, 67, 988-996.
[5] Khalifa, A.; Giles, M.; Ali, HI; Mohamady, S. Metal- and Catalyst-Free Synthesis of 2-Substituted-Phthalimides Using 2-(Arenesulfonyl)Phthalimide as Key Reagents[J]. Eur. J. Org. Chem. 2023, 26.
[6] Debenham, JS; Fraser-Reid, B. Tetrachlorophthaloyl as a Versatile Amine Protecting Group[J]. J.Org.Chem.1996,61,432-433.
[7] Astleford, B.; Weigel, LO "SCOPE OF PHTHALIMIDO CHEMISTRY I. EXTENSION OF UTILITY BY CONVERSION TO THE OPCB PROTECTING GROUP."[J]. Tetrahedron Lett., 1991, 32, 3301-3304.