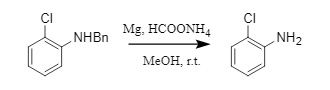1. Introduction
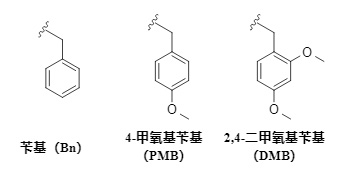
Common benzyl series protecting groups mainly include:
benzyl, 4-methoxybenzyl (PMB), 2,4-dimethoxybenzyl (DMB) (Formula 1). This series of protecting groups is usually relatively stable under alkaline conditions or in the presence of nucleophiles. Their introduction methods are similar, but their removal methods are diverse, mainly including catalytic hydrogenolysis, oxidation, and deprotection in an acidic environment.
(1)
2. Introduction Method
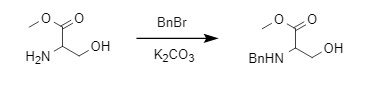
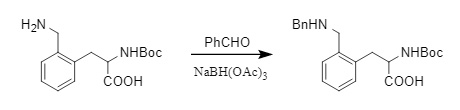
The most common method for introducing a benzyl protecting group is through reaction with the corresponding halide under alkaline conditions (Formula 2). Alternatively, the corresponding aldehyde can react with the amino group to form an imine, which can then undergo reductive amination in the presence of a reducing agent such as NaBH4 or NaBH(OAc)3 to yield the corresponding benzyl-protected product (Formula 3).
(2)
(3)
3. Removal method
There are many methods for removing benzyl groups, mainly including reductive debenzylation, oxidative debenzylation, acidic debenzylation, and alkaline debenzylation. Each of these methods has its own applicable scenarios and advantages, and disadvantages. They are classified and introduced as follows:
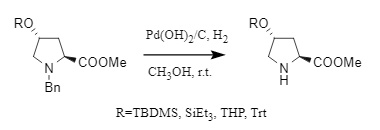
1. Reductive debenzylation: Reductive debenzylation mainly includes catalytic hydrogenation and transfer hydrogenation. The main difference between the two is the different hydrogen sources. Catalytic hydrogenation uses hydrogen gas as the hydrogen source, while the latter uses ammonium formate, cyclohexene, and other compounds to provide the hydrogen source. Commonly used catalysts include Pd/C and Pd(OH)2/C. In addition, metal magnesium and zinc have also been developed for debenzylation.
(1) Catalytic hydrogenation:
Catalytic hydrogenation is one of the most commonly used debenzylation methods (Formula 4). It has the advantages of mild reaction conditions, a clean system, and high yield, but its selectivity is poor, and it is easy to cause unnecessary side reactions on substrates that are easily reduced. In addition, during debenzylation, the use of acidic solvents or the addition of strong acids can promote the protonation of amines, preventing the amine compounds after debenzylation from interacting with the active sites of the catalyst and inhibiting the activity of the catalyst.
(4)
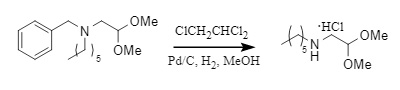
For small molecules and organic amines containing multiple hydrophilic groups, the debenzylation yield is often low due to stability and water solubility issues. Introducing polychlorinated alkanes into the system can significantly accelerate the debenzylation rate and achieve a one-pot conversion of debenzylation and salt formation.
(5)
In addition, the mixture of Pd/C and Pd(OH)2/C catalysts can achieve effects that cannot be achieved by a single catalyst, showing a more effective catalytic effect.
(2) Transfer hydrogenation:
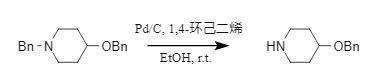
Transfer hydrogenation is an alternative method for catalytic hydrogenation. It does not require pressure equipment and is easy to operate. However, the introduction of hydrogen donors may cause trouble in the purification of products. The reagents used to provide hydrogen sources include formic acid, ammonium formate, cyclohexene, 1,4-cyclohexadiene, etc. Different hydrogen sources will affect the composition of the dehydrogenation product. For example, when 1,4-cyclohexadiene is used as a hydrogen source, it can selectively remove the N-benzyl group in N-, O-benzyl-containing substrates (Formula 6)
(6)
In addition, inexpensive metals such as magnesium and zinc can also be used as catalysts for transfer hydrogenation and debenzylation. For example, magnesium and ammonium formate can efficiently remove N-, O-, and S-benzyl groups, while substituents such as Boc, halogens, and carboxyl groups are not affected (Equation 7). This method avoids the high cost and spontaneous combustion of palladium catalysts and is a potential economical and environmentally friendly alternative for debenzylation.
(7)
2. Oxidative debenzylation: Oxidative debenzylation is a mild and highly selective method. Commonly used oxidizing agents include CAN (ceric ammonium nitrate) and DDQ (2,3-dichloro-5,6-dicyanobenzoquinone). CAN exhibits a certain degree of chemical inertness towards unsaturated bonds, allowing for selective debenzylation.
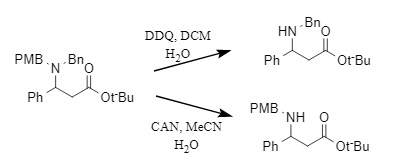
DDQ and CAN differ in their debenzylation selectivity (Equation 8). The former typically preferentially removes benzyl groups containing electron-donating groups, such as PMB, while the latter typically preferentially removes unsubstituted benzyl groups. CAN also exhibits good debenzylation efficiency for tertiary amines but is chemically inert towards secondary amines. Therefore, in polybenzyl substrates, CAN typically only removes a single benzyl group.
(8)
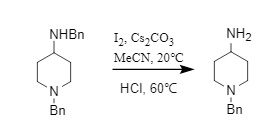
Oxidation systems such as O2/t-BuOK, I2/Cs2CO3, DIAD, and Oxone can all achieve benzyl removal. Among them, the I2/Cs2CO3 system can selectively remove the benzyl group of secondary amines while retaining the benzyl group of tertiary amines (Formula 9); DIAD can selectively remove the N-benzyl group, while the O-benzyl group can exist stably.
(9)
3. Acidic debenzylation:
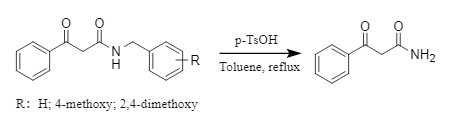
Acidic debenzylation is also a fast and simple debenzylation method, but this method requires the substrate to have a certain tolerance to an acidic environment. Commonly used acid decomposition reagents include trifluoroacetic acid (TFA), trifluoromethanesulfonic acid (TfOH), and p-toluenesulfonic acid (p-TsOH), which are usually used to remove benzyl groups containing electron-donating groups (PMB, DMB, etc.) (Formula 10)
(10)
4. Alkaline debenzylation
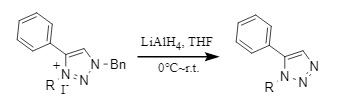
is often used as an alternative to catalytic hydrogenolysis and is effective for the debenzylation of N-acyl-N-benzyl derivatives, aromatic heterocycles, and other compounds (Formula 11). The reagents used include alkyl lithium reagents (MeLi, etc.), Na/NH3(l), LiAlH4, etc.
(11)
5. Other Methods:
In addition to the common debenzylation methods mentioned above, numerous other debenzylation methods, such as enzyme catalysis, nitrolysis, Grignard reagents, and NBS/AIBN, can also achieve efficient debenzylation in specific scenarios and serve as a supplement to conventional debenzylation methods. These methods will not be discussed in detail here.
In summary, each of the aforementioned debenzylation methods has its own advantages and disadvantages and suitable scenarios. In complex organic synthesis reactions, the appropriate debenzylation strategy should be selected based on the structural characteristics of the specific reaction substrate.
References:
[1] Liu Qiaozhen, Jiang Fuxiang, Wang Guo, Chen Heru. Efficient removal of benzyl protecting groups from nitrogen-containing sugars by Pd/C catalytic hydrogenation[J]. Journal of Jinan University (Natural Science Edition), 2013, 34, 319-323.
[2] Zhou Guangwei, Zhang Lizhu, Xue Yahan, Li Jiarong. Research progress in N-benzyl removal[J]. Organic Chemistry, 2019, 39,2428-2442.
[3] Guo Yanhao, Hao Qinghui, Hao Siyuan, et al. Protection and deprotection of amino groups in chemical products[J]. Coal and Chemical Industry, 2022, 45, 106-112.
[4] Park, JD; Kim, DH Cysteine Derivatives as Inhibitors for Carboxypeptidase A: Synthesis and Structure-Activity Relationships[J]. J. Med. Chem., 2002, 45, 911-918.
[5] Dai Yunsheng, Dong Shouan, Pan Zaifu, Chen Jialin. Research and application of catalytic hydrogenolysis of benzyl groups on Pd/C catalyst[J]. Industrial Catalysis, 2011, 19, 7-10.
[6] Babu, SNN; Srinivasa, GR; Santhosh, DC; Gowda, DC The hydrogenolysis of N-benzyl groups with magnesium and ammonium formate[J]. J. Chem. Res., 2004, 1.