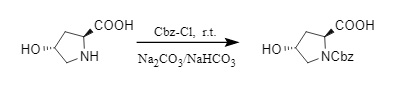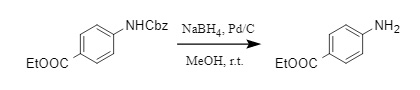1. Introduction
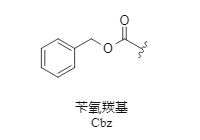
Cbz is one of the most commonly used amino protecting groups (1). Its advantage is that it is easy to introduce and remove. In addition, the introduction of the Cbz protecting group usually makes the protected product easy to crystallize and has improved stability, making it easy to purify by crystallization.
(1)
2. Introduction Method
1. Alkaline conditions:
(1) There are many methods for introducing Cbz protecting groups. The most commonly used method is to react with benzyl chloroformate (Cbz-Cl) under alkaline conditions to generate N-Cbz protected amino compounds (2). In addition to Cbz-Cl, activated esters such as N-benzylsuccinimidyl carbonate Cbz-Osu and 4-nitrophenyl benzyl carbonate (Cbz-ONB) can also be used as reagents for introducing benzyloxycarbonyl groups.
(2)
(2) When using Cbz-Cl to introduce Cbz under alkaline conditions, the pH is usually controlled between 8 and 10. A pH that is too low will cause Cbz-Cl to decompose, while a pH that is too high may cause amino acid racemization. Therefore, the base not only acts as an acid-binding agent to neutralize the generated HCl in this process, but also maintains the pH stability of the reaction system, which is somewhat difficult in large-scale production. The Abell team developed a mixed base buffer system of Na2CO3: NaHCO3 = 2:1, which can effectively maintain the pH in the range of 8-10 and is suitable for Cbz protection of various chiral amino acids (3).
(3)
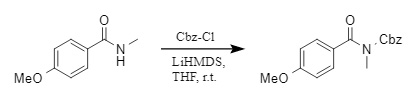
(3) In addition, Sureshbabu's group developed a convenient method to introduce Cbz protecting groups on the nitrogen of secondary amides (4). In the presence of the base LiHMDS (lithium bis(trimethylsilyl)amide), the secondary amide reacted with Cbz-Cl to successfully achieve N-Cbz protection.
(4)
2. Non-alkaline conditions:
Cbz protecting groups can also be used to mildly and efficiently protect N-Cbz under non-alkaline conditions.
(1) Cbz-Cl/I2/MeOH:
Varala's team developed an iodine-catalyzed method for the efficient introduction of Cbz protecting groups. A catalytic amount of iodine acts as a Lewis acid, gently and efficiently introducing Cbz protecting groups into amine compounds with significant differences in structure and electronic properties (5)
(5)
(2) Cbz-Cl/PEG-600:
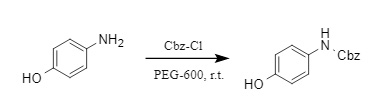
Professor Huang Haihong's team developed an efficient, environmentally friendly and chemically selective method for introducing Cbz protecting groups (6). This method uses green and low-toxic PEG-600 as the reaction medium. Aliphatic amines and aromatic amines react with Cbz-Cl to obtain the corresponding N-Cbz derivatives in high yields.
(6)
3. Removal method
There are various methods for removing the Cbz protecting group, with catalytic hydrogenolysis being the most common method. When the substrate contains other sensitive groups, selective deprotection under acidic, alkaline, or other conditions can be considered.
1. Catalytic hydrogenolysis:
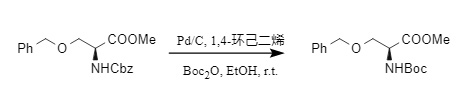
Catalytic hydrogenolysis includes catalytic hydrogenation and transfer hydrogenation, both of which are suitable for the removal of Cbz. Normally, when there is sufficient hydrogen source, carbon dioxide and toluene will be generated; when there is insufficient hydrogen source, a side reaction will occur, generating N-benzyl-protected tertiary amines. If Boc2O is also present in the system, N-Cbz protection can be converted into N-Boc derivatives in one step, and easily reducible functional groups such as benzyl ether will not be affected (7).
(7)
In addition, the combination of Pd-C/NaBH4/MeOH is also a convenient method for removing Cbz (8). This method can quickly and simply remove Cbz by generating hydrogen on site and has good compatibility with many conventional protecting groups.
(8)
2. Acidic conditions:
The Cbz protecting group can also be easily removed under acidic conditions. HBr/HOAc is a commonly used Cbz removal system (9). The deprotection rate increases with increasing HBr concentration.
(9)
Similarly, Lewis acids can also be used to remove N-Cbz protection. The AlCl3/HFIP (hexafluoroisopropanol) system can selectively remove Cbz protection mildly and safely in the presence of sensitive groups such as nitro groups, double bonds, and benzyl groups, and has good substrate applicability (10).
(10)
3. Alkaline conditions:
Under certain specific conditions, alkaline conditions can also achieve the removal of Cbz protecting groups. For example, in N, N'-bis-Cbz-protected diazinoic acid compounds, one Cbz protecting group can be selectively removed under alkaline conditions (11). In addition, in a high concentration of sodium hydroxide solution, the Cbz protecting group at a specific position of kanamycin A intermediate, such as the N-3' position, can be selectively removed.
(11)
4. Other conditions:
In addition to the deprotection methods mentioned above, many other methods can be used to remove N-Cbz protection under specific conditions. For example, lower alcohols (methanol, ethanol, etc.) can be used to remove N-Cbz protecting groups on imidazole and pyrazole compounds (12). Organometallic reagents such as the n-Bu3SnH/AIBN system can selectively remove N-Cbz protecting groups on amides and nitrogen-containing heteroaromatic rings, while Cbz protection on aliphatic amines is not affected. These methods can serve as supplements to conventional Cbz removal methods and will not be described in detail in this article.
(12)
In summary, the above-mentioned Cbz removal methods each have their own advantages and disadvantages, and are suitable scenarios. In complex organic synthesis reactions, the introduction and removal of protecting groups require the selection of appropriate synthetic strategies based on the structural characteristics of the specific reaction substrates.
References:
[1] Xu Qiuxia. Research on the chemical synthesis of glutathione [D]. Donghua University, 2010.
[2] Pehere, AD; Abell, AD. An improved large-scale procedure for the preparation of N-Cbz amino acids [J]. Tetrahedron Lett., 2011, 52, 1493-1494.
[3] Sureshbabu, P.; Azeez, S.; Kandasamy, J., et al. Synthesis of N-Cbz Amides and Their Applications in the Transamidation Reactions at Room Temperature [J]. Asian J. Org. Chem., 2022, 11.
[4] Varala, R.; Enugala, R.; Adapa, SR. Molecular Iodine-Catalyzed Efficient N-Cbz Protection of Amines [J]. J. Iran. Chem. Soc., 2007, 4, 370-374.
[5] Zhang, CL; Zhang, DF; Huang, HH; et al. A facile protocol for N-Cbz protection of amines in PEG-600 [J]. Chinese Chemical Letters, 2012, 23, 789-792.
[6] Cameron, M.; Wilson, R.D. The unexpected formation of N-benzylated tertiary amines from their corresponding CBZ-protected precursors [J]. APPL CATAL A-GEN, 2000, 203, 307-310.
[7] Bajwa, JS One-Pot Transformation of Benzyl Carbamates into t-Butyl Carbamates[J]. Tetrahedron Lett. 1992, 33, 2955-2956.
[8] Sultane, PR; Mete, TB; Bhat, RG. A Convenient Protocol for the Deprotection of N-Benzyloxycarbonyl (Cbz) and Benzyl ester groups[J]. Tetrahedron Lett., 2015, 56, 2067-2070.
[9] Vinayagam, V.; Sadhukhan, SK; Kumar, TVH; et al. Mild Method for Deprotection of the N Benzyloxycarbonyl (N Cbz) Group by the Combination of AlCl3 and HFIP[J]. J. Org. Chem., 2024, 89, 5665-5674.
[10] Papadaki, E.; Georgiadis, D.;
[11] Chen, GH; Pan, P.; Li, ZJ; et al. Selective deprotection of the Cbz amine protecting group for the facile synthesis of kanamycin A dimers linked at N-300 position[J]. Tetrahedron, 2009, 65, 5922-5927.
[12] Song, GQ; Huang, XF; Yang, B.; et al. Easy Removal of N-carboxybenzyl (Cbz) Protective Group by Low Carbon Alcohol[J]. Lett. Org. Chem., 2016, 13, 177-180.
[13] Bennasar, ML; Roca, T.; Padullés, A. Chemoselective Radical Cleavage of Cbz-Protected Nitrogen Compounds[J]. Org. Lett., 2003, 5, 569-572.