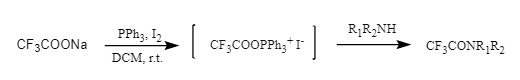1. Introduction
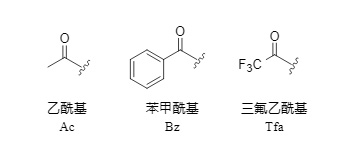
Monoacyl groups are a diverse class of amino-protecting groups that are widely used in peptide and drug synthesis. Among them, acetyl, benzoyl, and trifluoroacetyl are the most commonly used (Figure 1). Trifluoroacetyl, in particular, is highly favored in peptide synthesis due to its convenient removal method, which is well-suited to orthogonal protection strategies.
Figure 1: Common monoacyl protecting groups
Acetyl and benzoyl groups lack strong electron-withdrawing groups and usually require severe hydrolysis conditions to be removed, which can easily cause racemization of polypeptides and removal of other protecting groups. Therefore, their application range is subject to certain limitations.
2. Non-halogenated acyl groups
Due to the strong electron-withdrawing property of trifluoroacetyl, its properties are significantly different from those of benzoyl and acetyl. Therefore, this article classifies benzoyl and acetyl as non-halogenated acyl groups and describes them uniformly.
1. Introduction method
(1) Conventional method:
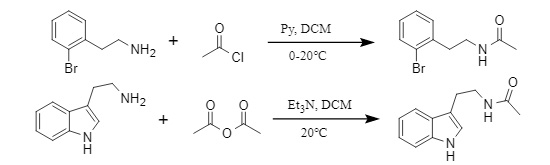
Conventional acyl introduction methods usually use acid anhydride/acyl chloride to introduce acyl groups by acylation in the presence of a base, or introduce acyl groups by coupling reaction of carboxylic acid and amine under the promotion of a condensation reagent. Taking acetyl as an example (Figure 2), the introduction methods of acetyl chloride and acetic anhydride are as follows:
Figure 2: Method of introducing acetyl groups
(2) Special methods:
① Amide method
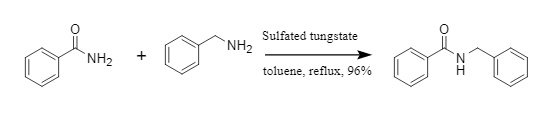
Pathare et al. developed a transamidation reaction catalyzed by modified tungstate. Through the catalysis of modified tungstate, amides and amine compounds are reacted to convert into new amide compounds (Figure 3). This method applies to a variety of aromatic amides and aliphatic amides, with mild reaction conditions and high efficiency.
Figure 3: Transamidation reaction catalyzed by modified tungstate
②N-Acylbenzotriazole method
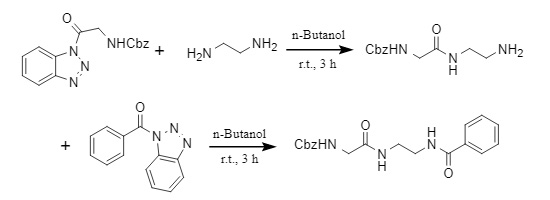
Agha et al. developed a new acyl introduction method. Using acylbenzotriazole as an acylating agent, they can selectively monoacylate unprotected aromatic/aliphatic diamines in green solvents such as water and n-butanol. At the same time, they can also gradually acylate aliphatic diamines to synthesize asymmetric diamides (Figure 4).
Figure 4: Synthesis of asymmetric diamide
2. Deacylation method:
The stability of the amide bond is relatively high, and deacylation under mild conditions is more difficult. Compared with the hydrolysis of the ester group, the hydrolysis of the amide group often requires more intense conditions.
(1) Conventional method:
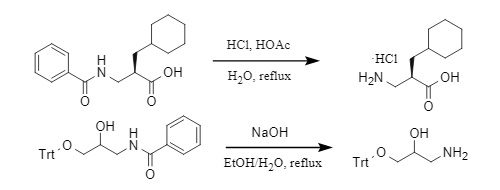
Generally, the acyl group can only be removed by high-temperature reaction under strong acidic/alkaline conditions. Taking benzoyl as an example (Figure 5), deacylation is carried out under acidic and alkaline conditions, respectively:
Figure 5: Method for removing the benzoyl group
(2) Special methods:
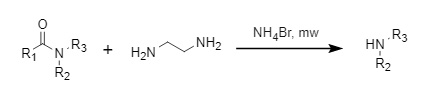
① Ammonium bromide method
Shimizu et al. developed a microwave-assisted deacylation method using the synergistic action of ammonium bromide and ethylenediamine, which has a high yield and is compatible with a variety of functional groups (Figure 6).
Figure 6: Synergistic deacylation of ammonium bromide and ethylenediamine
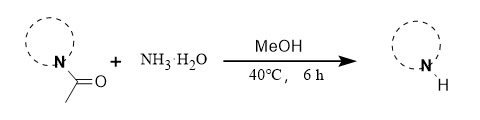
② Ammonia method:
Professor Zeng's team developed a mild deacylation method using ammonia as the deacylation reagent. The reaction conditions are mild, and the yield is high. It applies to a series of compounds such as various substituted indoles (Figure 7).
Figure 7: Ammonia deacylation reaction
3. Haloacyl
Haloacetyl groups are more easily removed under the influence of halogen atoms. Among them, the representative protecting group trifluoroacetyl can be removed under mild alkaline conditions and is widely used in peptide and drug synthesis.
1. Introduction method
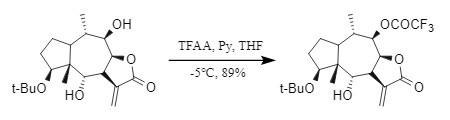
(1) Trifluoroacetic anhydride (TFAA): Trifluoroacetic anhydride is highly active and usually requires a lower temperature and the presence of a base (Figure 8). It is a common trifluoroacetylation reagent, but when it is introduced into Tfa, it is easy to cause side reactions such as racemization of the chiral center or peptide bond cleavage.
Figure 8: Trifluoroacetic anhydride introduced into Tfa
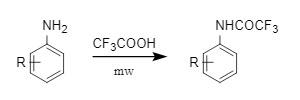
(2) Trifluoroacetic acid (TFA): Compared with trifluoroacetic anhydride and trifluoroacetyl chloride, trifluoroacetic acid has a higher boiling point and a wider range of applications. It can be used to trifluoroacetylate substituted anilines under microwave-assisted conditions (Figure 9). It can also be introduced through a classic carboxylic acid-amine condensation reaction or directly trifluoroacetylated aromatic amines by heating in xylene.
Figure 9: Trifluoroacetic acid introduced into Tfa
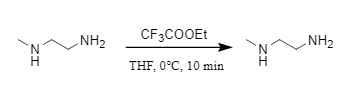
(3) Ethyl trifluoroacetate: Ethyl trifluoroacetate is commonly used in peptide synthesis and can be achieved by reacting with amines at 0°C in THF. Under the influence of steric hindrance, this reagent can selectively protect primary amines in the presence of secondary amines (Figure 10). Even for the same primary amine, different alkyl groups will have different reaction effects. For example, in the presence of tert-butylamine and isobutylamine, it will preferentially react with isobutylamine, which has less steric hindrance (accounting for 98%).
Figure 10: Introduction of TFA into ethyl trifluoroacetate
(4) Acyloxyphosphonium salts: Iodine/bromide acyloxyphosphonium salts were prepared by sodium trifluoroacetate/triphenylphosphine/iodine or trifluoroacetic acid/triphenylphosphine/NBS, and then attacked by amines to give trifluoroacetamides (Figure 11).
Figure 11: Introduction of acyloxyphosphonium salt into Tfa
(5) Other reagents:
Many of the introduced reagents are derivatives of trifluoroacetic acid/trifluoroacetic anhydride. By synthesizing derivatives with easy-leaving groups, such as acyl imidazole, acyl benzotriazole, etc., they react with amines to obtain the corresponding amides.
2. Removal methods:
The removal method of trifluoroacetyl is relatively mild and relatively stable under strong acidic conditions. It is compatible with protecting groups such as Boc, Z, Trt, and Alloc. The following removal methods are commonly used:
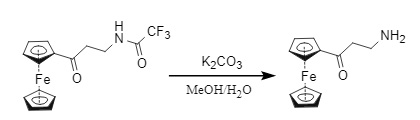
(1) Na2CO3 (K2CO3)/MeOH/H2O (Figure 12)
Figure 12: Carbonate removal of Tfa
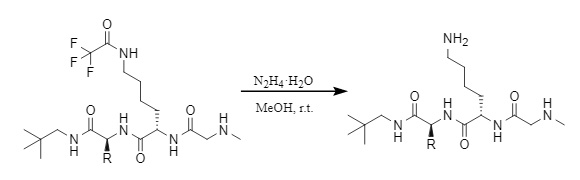
(2) Hydrazine hydrate/methanol
Figure 13: Removal of Tfa by hydrazine hydrate
(3) Other methods:
In addition to the above deacylation methods, there are several mild removal methods, including NaOH aqueous solution, 1 M piperidine aqueous solution, NaBH4/EtOH, and other methods.
References:
[1] Guo Yanhao, Hao Qinghui, Hao Siyuan, et al. Protection and deprotection of amino groups in chemical products [J]. Coal and Chemical Industry, 2022, 45, 106-112.
[2] Gao Xuhong, Li Bingqi. Amino protection and application in organic synthesis (review) [J]. Journal of Shihezi University (Natural Science Edition), 1999, 3, 76-86.
[3] Balieu, S.; Toutah, K.; Rousselière, H.; et al. Radical cyclization of ynamides into six- or eight-membered rings. Application to the synthesis of a protoberberine analog [J]. Tetrahedron Lett., 2011, 52, 2876-2880.
[4] Warren, HT; Saeger, HN; Rasmussen, K.; et al. Psychoplastogenic DYRK1A Inhibitors with Therapeutic Effects Relevant to Alzheimer's Disease[J]. J. Med. Chem., 2024, 67, 6922−6937.
[5] Pathare, AP; Jain, AKH; Akamanchi, KG Sulfated tungstate: a highly efficient catalyst for transamidation of carboxamides with amines[J]. RSC Adv., 2013, 3, 7697-7703.
[6] Agha, KA; Abo-Dya, ND, Abdel-Samii, ZK; et al. N Acylbenzotriazole: convenient approach for protecting group-free monoacylation of symmetric diamines[J]. Monatsh. Chem., 2020, 151, 589–598.
[7] Doboszewski, B., Groaz, E., Herdewijn, P. Synthesis of Phosphonoglycine Backbone Units for the Development of Phosphono Peptide Nucleic Acids[J]. Eur. J. Org. Chem., 2013, 4804–4815.
[8] Benkel, T.; Annala, S.; Gütschow, M.; et al. BIM-46174 Fragments as Potential Ligands of G Proteins[J]. Med. Chem. Commun., 2019, 00, 1-3.
[9] Shimizu, Y.; Morimoto, H.; Zhang, M.; Ohshina, T. Microwave-Assisted Deacylation of Unactivated Amides Using Ammonium-Salt-Accelerated Transamidation[J]. Angew. Chem. Int. Ed. 2012, 51, 8564–8567.
[10] Han Qun, Xu Kun, Zeng Chengchu et al. A practical method for removing acyl protecting groups by amidotransfer reaction[J]. Organic Chemistry, 2022, 42, 1123-1128.
[11] López, SE; Restrepo, J.; Salazar, J. Trifluoroacetylation in Organic Synthesis: Reagents, Developments and Applications in the Construction of Trifluoromethylated Compounds[J]. Curr. Org. Synth., 2010, 7, 414-432.
[12] Piotrowicz, M.; Maslowska, N.; Rudolf, B.; et al. Synthesis of Trifluoroacetamidoketones by Acylation of Ferrocene with In Situ Protected Amino Acids[J]. J. Org. Chem., 2025, 90, 2958−2968.
[13] Heller, P.; Weber, B.; Birke, A.; Barz, M. Synthesis and Sequential Deprotection of Triblock Copolypept(o)ides Using Orthogonal Protective Group Chemistry[J]. Macromol. Rapid Commun., 2015, 36, 38−44.
[14] Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino Acid-Protecting Groups[J]. Chem. Rev.., 2009, 109, 2455–2504.