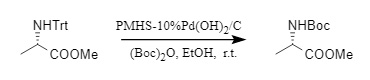Trityl protective groups present steric hindrances, offer mild conditions for introduction and removal, are easy to operate, and exhibit high stability, making them particularly suitable for the selective protection of multifunctional compounds. Furthermore, the introduction of trityl protective groups facilitates crystallization, thereby facilitating purification by crystallization.
1. Introduction
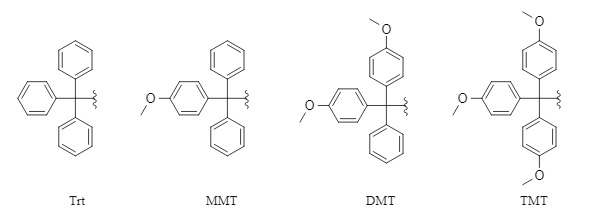
Common trityl protecting groups include
trityl (Trt), 4-methoxytrityl (MMT), 4,4'-dimethoxytrityl (DMT), and 4,4',4"-trimethoxytrityl (TMT) (Figure 1). This series of protecting groups is relatively stable under alkaline conditions or in the presence of nucleophiles, but is easily removed under acidic conditions. The removal activity under acidic conditions is in the order of TMT > DMT > MMT > Trt. Given that the introduction and removal methods of this series of protecting groups are generally similar, this article focuses on Trt as an example.
Figure 1: Common trityl series protecting group structures
2. Introduction Method
Due to their significant steric hindrance, trityl protecting groups are commonly used to protect primary amines. Their functionality is similar to that of ethyl trifluoroacetate and N-ethoxycarbonylphthalimide, enabling selective protection of primary amines. Furthermore, in peptide synthesis, trityl protection can effectively inhibit racemization during the reaction, making it particularly suitable for peptides containing histidine.
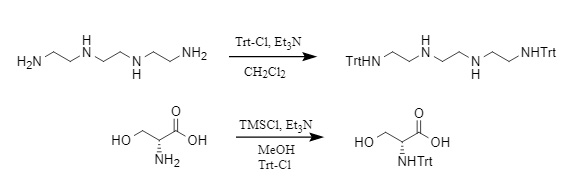
The Trt protecting group is typically introduced using Trt-Cl under alkaline conditions, which is one of the most common methods. Other methods include the use of Trt-OH (trityl alcohol) and Ac2O under acidic conditions, or the use of a combination of TMSCl/Et3N/Trt-Cl to introduce the trityl group. Examples are shown in Figure 2:
Figure 2: Example of trityl introduction
3. Removal method
Although the trityl group is relatively stable, it is an acid-sensitive group and is easily removed under various acidic conditions, such as hydrochloric acid, TFA, and acetic acid. The Boc protecting group is also typically removed under acidic conditions, allowing for selective removal based on the acid sensitivity of different protecting groups. For example, Trt can be easily removed in a 50% aqueous HOAc solution, while the Boc protecting group remains unaffected. Sieber et al. investigated the stability of the Trt and Boc protecting groups in Trt-His(Trt)-Lys(Boc)-OMe (Figure 3) and found that the Trt protecting group on the histidine side chain is more stable than the Trt protecting group on the α-amino group. Furthermore, in a 1 N HCl/acetic acid system, the Boc protecting group is removed while the Trt protecting group on the histidine side chain remains unaffected.
Figure 3 Study on the stability of Trt and Boc protecting groups in Trt-His(Trt)-Lys(Boc)-OMe
In addition, the trityl group can be deprotected by the following methods:
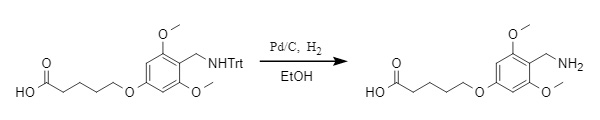
1. Reductive removal: The Trt protecting group can be removed under reducing conditions such as Pd/C hydrogenation (see figure below) and Na/NH3(l). Under catalytic hydrogenation, the removal rate of Trt is significantly lower than that of other protecting groups such as O-Bn and N-Cbz, allowing for selective removal based on the characteristics of different protecting groups.
Figure 4 Pd/C catalytic hydrogenation for Trt removal
In addition, the Chandrasekhar team developed a method to convert N-Trt protection into N-Boc protection in one step. Through the action of Pd(OH)2/C and PMHS (polymethylhydrogensiloxane), N-Boc compounds can be synthesized in a single step with high efficiency and a wide substrate range.
Figure 5: One-step synthesis of N-Boc protection
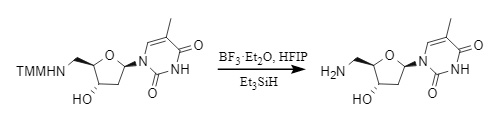
2. Lewis acid removal: For substrates sensitive to proton acids, Lewis acids such as ZnBr2, BF3·Et2O, etc., are effective deprotection reagents. For example, in the BF3·Et2O/Me3SiH/HFIP (hexafluoroisopropanol) system, rapid and efficient removal is possible:
Figure 6 Lewis acid demethylation of MMT
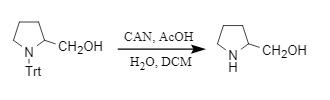
3. CAN (cerium ammonium nitrate) reagent removal: CAN reagent can remove Trt by single electron transfer, thereby converting the amino group into an amino anion. In the process, acetic acid and other acids are required to provide hydrogen protons to obtain free amines; otherwise, the raw materials will be regenerated.
Figure 7: Trt removal by CAN reagent
Trityl protecting groups are widely used in nucleoside and peptide synthesis due to their ease of introduction and removal. While steric hindrance provides them with good selectivity, it also limits certain reactions: most Trt-amino acids are difficult to construct amide bonds using the mixed anhydride or azide methods, and Trt-amino acid esters are also difficult to hydrolyze due to their significant steric hindrance.
Therefore, after gaining a deep understanding of the characteristics of various protecting groups, researchers can select the most appropriate protection strategy based on specific reaction conditions and molecular structure characteristics to efficiently synthesize target compounds.
References:
[1] Zhang Panpan, Zheng Tucai, Chen Sheng, et al. Progress in the application of trityl protecting groups in organic synthesis[J]. Journal of Synthetic Chemistry, 2014, 2, 28-40.
[2] Ito, C.; Taguchi, K.; Matsumoto, K.; et al. Trimethoxy Trityl Groups as a Potent Substituent for Anti-cancer Cytidine Analog Prodrugs[J]. J. Pharm. Sci., 2022, 111, 2201-2209.
[3] Sieber, P.; Riniker, B. Protection of carboxamide functions by the trityl residue. Application to peptide synthesis[J]. Tetrahedron Lett., 1991, 32, 739-742.
[4] Krakowiak, KE; Bradshaw, JS Selective Protection of the Primary Amine Functions of Linear Tetraamines Using the Trityl Group[J]. Synth. Commun., 1998, 28, 3451-3459.
[5] Baghery, S.; Zarei, M.; Behranvand, V.; et al. Application of trityl moieties in chemical processes: Part I[J]. J. Iran. Chem. Soc., 2020, 17, 2737-2843.
[6] Sieber, P.; Riniker, B. Protection of histidine in peptide synthesis: A Reassessment of the trityl group[J]. Tetrahedron Lett., 1987, 28, 6031-6034.
[7] Sharma, SK; Songster, MF; Castellino, FJ; et al. Reductive Amination with Tritylamine as an Ammonia Equivalent: Efficient Preparation of the 5-[4-[(9-Fluorenylmethyloxycarbonyl)- amino]methyl]-3,5-dimethoxyphenoxy]valeric Acid (PAL) Handle for Peptide Synthesis[J]. J. Org. Chem., 1993, 58, 4993-4996.
[8] Chandrasekhar, S.; Babu, BN; Reddy, CR Single-step conversion of N-benzyl, N-trityl and N-diphenylmethyl amines to t-butyl carbamates using polymethylhydrosiloxane[J]. Tetrahedron Lett., 2003, 44, 2057-2059.
[9] Bege, M.; Bereczki, L.; Herczegh, P.; et al. A three-component reagent system for rapid and mild removal of O-, N- and S-trityl protecting groups[J]. Org. Biomol. Chem., 2016, 1–3.
[10] Pattanayak, S.; Sinha, S. Ceric ammonium nitrate-mediated detritylation of tritylated amines[J]. Tetrahedron Lett., 2011, 55, 34-37.