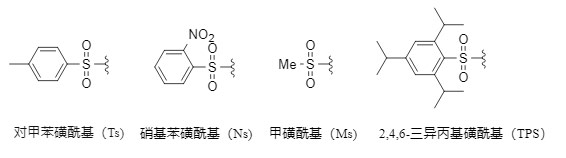
It should be noted that different protecting groups have different tolerance conditions. Among all amino protecting groups, the sulfonyl group (Figure 1 shows several common sulfonyl protecting groups) is one of the most stable protecting groups. Its introduction method is generally to react amine with corresponding sulfonyl chloride in an inert solvent under alkaline conditions. It has the following advantages:
(1) It is compatible with a variety of reaction conditions and is not affected by treatment under strong base, hydrogenation, and other conditions. Moreover, the product after the introduction of sulfonyl group can easily form crystals, which is convenient for acquisition and purification.
(2) The introduction of the sulfonyl group makes the NH bond more active so that the N-alkylation reaction and transition metal-catalyzed CN formation can easily occur.
Figure 1. Common sulfonyl protecting groups
Similarly, the higher stability of the sulfonyl group also makes its deprotection conditions more stringent. Acidic hydrolysis usually has to be carried out under severe conditions, such as heating with concentrated sulfuric acid; a milder method is through reductive cleavage, such as Na/NH3(l), Mg/MeOH, and other systems. However, this method is limited to substrates containing easily reducible groups such as aldehydes, ketone carbonyls, and nitro groups, and is prone to side reactions.
This article introduces a method based on stoichiometric trifluoromethanesulfonic acid to effectively remove the protection of aromatic amine sulfonyl groups and related applications.
1. Excellent substrate universality:
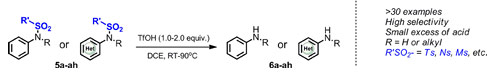
At room temperature or under heating conditions, trifluoromethanesulfonic acid can effectively remove the protecting groups of unsubstituted and N-substituted aromatic/heteroaromatic sulfonamide substrates to obtain the corresponding aromatic amines in high yields. Although the reaction is carried out under acidic conditions, it can tolerate substrates containing cyano, imide, ester groups, as well as basic substrates such as pyridine and quinoline. This method is applicable to protecting groups such as p-toluenesulfonyl (Ts), nitrobenzenesulfonyl (Ns), methylsulfonyl (Ms), and triisopropylsulfonyl (TPS) (Figure 2).
Figure 2 Trifluoromethanesulfonic acid-promoted sulfonamide deprotection reaction
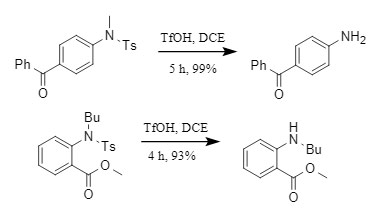
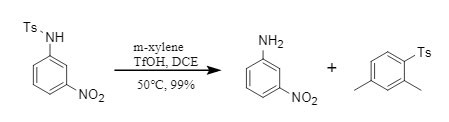
① Neutral and electron-deficient substrates: The NS bond in sulfonamide is easily broken, achieving efficient removal of the sulfonyl group (Figure 3);
Figure 3 Deprotection reaction of electron-deficient substrates
② Electron-rich substrates: Fries rearrangement (intramolecular sulfonyl migration) occurs to generate a series of sulfone compounds (Figure 4).
Figure 4 Deprotection reaction of electron-rich substrates
2. Selective removal of the Ts protecting group:
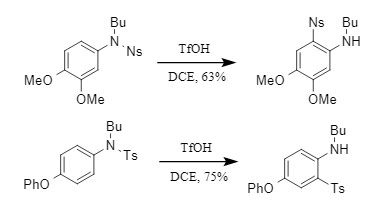
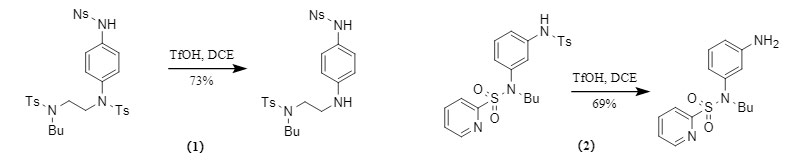
Normally, it is difficult to remove the Ts protecting group. However, the Orentas team found in the experiment that in the presence of TfOH, Ts can be selectively removed without affecting other sulfonyl protecting groups (Figure 5).
① Ts protecting groups on aromatic amines are preferentially removed: There are differences in the stability of Ts protecting groups at different positions, among which the Ts protecting groups on aromatic amines are easier to remove;
② Excellent selectivity: When Ts coexists with other sulfonyl protecting groups such as Ns and Ms, the Ts protecting group can still be selectively removed. Even when the very unstable pyridine-2-sulfonyl coexists with Ts, the Ts protecting group is still preferentially removed.
Figure 5. Selective removal of Ts protecting group
3. Other applications:
① Intermolecular sulfonyl transfer: In the presence of electron-rich aromatic compounds, electron-deficient sulfonamide substrates can undergo FC sulfonylation (here refers to intermolecular sulfonyl transfer) while being deprotected to generate sulfone compounds (Figure 6).
Figure 6. Intermolecular migration of sulfonyl groups
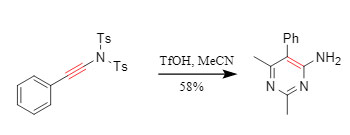
② One-pot cyclization: Conjugated sulfonamide substrates can undergo a [2+2+2] cycloaddition reaction while being deprotected. For example, acetonitrile and acetonitrile undergo a nitrile cyclization reaction in the presence of TfOH to obtain aminopyrimidines in one pot (Figure 7).
Figure 7. Cyclization reaction involving sulfonamide
This article introduces new methods for removing sulfonyl groups and their expanded applications, hoping to provide help and ideas for researchers.
References:
[1] Orentas, E.; Javorskis, T. Chemoselective Deprotection of Sulfonamides Under Acidic Conditions: Scope, Sulfonyl Group Migration, and Synthetic Applications [J]. J. Org. Chem., 2017, 82, 13423-13439.
[2] Kisla, MM; Kul, P.; Kus, C.; et al. Incorporation of Protecting Groups in Organic Chemistry: A Mini-Review [J]. Curr. Org. Synth., 2023, 20, 491-503.
[3] Guo Yanhao, Hao Qinghui, Hao Siyuan, et al. Protection and deprotection of amino groups in chemical products [J]. Coal and Chemical Industry, 2022, 45, 106-112.