12/9/2021
Dibromotriphenylphosphine was first used in the synthesis reaction in 1959 to prepare bromide and acid bromide from alcohol and carboxylic acid, and it was also used in the dehydration reaction of amides and oximes to nitriles. Now it has been used as a general reagent in many reactions
1 Application of dibromotriphenylphosphine
Dibromotriphenylphosphine is often used as a brominating agent in organic synthesis, and its structural formula is as follows:
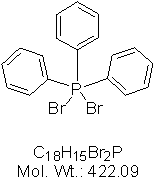
Dibromotriphenylphosphine was first used in the synthesis reaction in 1959 to prepare bromide and acid bromide from alcohol and carboxylic acid, and it was also used in the dehydration reaction of amides and oximes to nitriles. Now it has been used as a general reagent in many reactions.
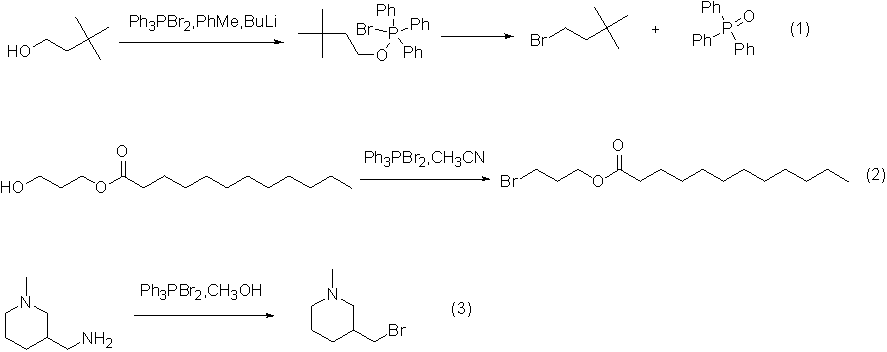
Application 1: Alcohol Synthesis of Bromide
The reaction between dibromotriphenylphosphine and alcohol first generates alkoxytriphenylphosphine bromide intermediate, which reacts very quickly, and then the intermediate slowly decomposes into triphenylphosphine oxide and corresponding bromide. The by-product triphenoxyphosphine can be removed by distillation or beating, and the yield is relatively high.
An example of the reaction is shown in the figure below:
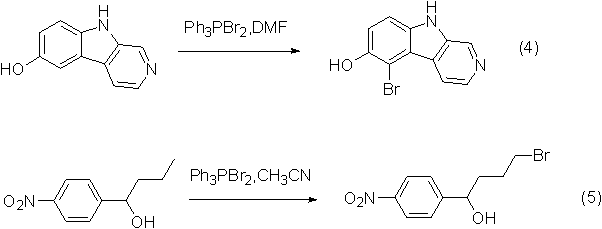
Application 2: bromination reaction
Dibromotriphenylphosphine is also a good bromination reagent, which can replace bromine in many reactions and enhance the operability of the reaction.
An example of the reaction is shown in the figure below:
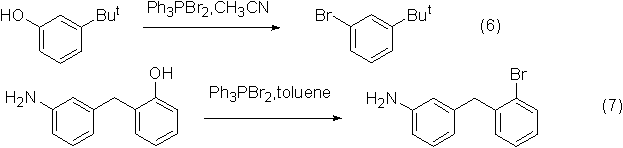
Application 3: Synthesis of aryl bromide
The reaction of dibromotriphenylphosphine and phenol can prepare brominated aromatic compounds, but the reaction yield is low.
An example of the reaction is shown in the figure below:
Application 4: The reaction of ring-opening of epoxides to form o-dibromo compounds
Epoxides and dibromotriphenylphosphine undergo ring-opening reactions in toluene to form o-dibromo compounds.
An example of the reaction is shown in the figure below:
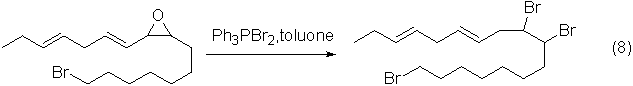
Application 5: Preparation of bromoalkenes by reacting with ketones
The reaction of dibromotriphenylphosphine and ketone compound to prepare brominated unsaturated compound has a higher yield.
An example of the reaction is shown in the figure below:
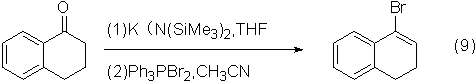
The two-step total yield of the reaction can reach 54%.
Application 6: Reaction with carboxylic acid and acid anhydride to generate acid bromide
Acyl bromide can be prepared by reacting dibromotriphenylphosphine with carboxylic acid or acid anhydride, and the yield of this type of reaction is relatively high.
An example of the reaction is shown in the figure below:
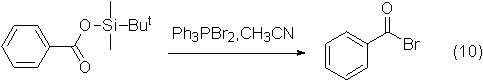
The reaction yield can reach 89%.
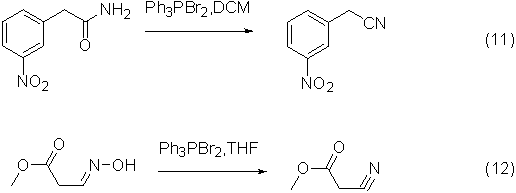
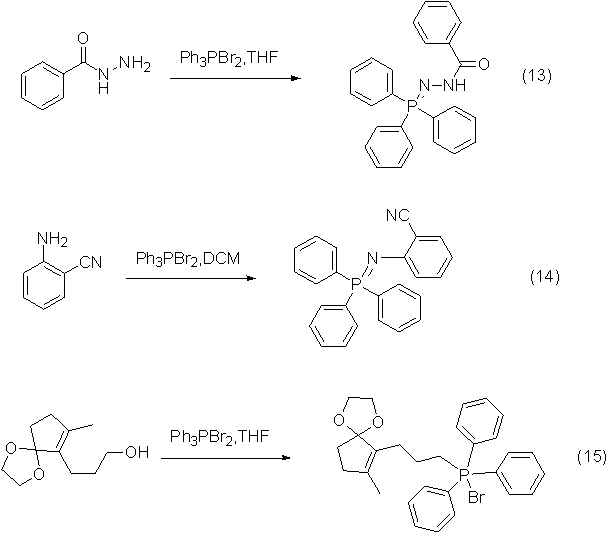
Application 7: Dehydration reaction of amides and oximes to nitriles
Dibromotriphenylphosphine can also be used as a dehydrating agent to dehydrate amides and oximes to form nitriles, and the reaction effect is very good.
An example of the reaction is shown in the figure below:
In addition to the above applications, dibromotriphenylphosphine can also participate in the following reactions:
references:
1. Jeffery, K.; Slayton, AWEJ Org. Chem., 1986, 51, 5490.
2. Chavan, SP; Kharul, RK; Kamat, SK; Kalkote, UR;Kale, RR Synth. Commun., 2005, 35, 987.
3. Menger, FM; Galloway, ALJ Am. Chem. Soc., 2004, 126, 15883.
4. Czarnik, AWJ Org. Chem., 1984, 49, 924.
5. Kamei, K.; Maeda, N.; Tatsuoka, T. Tetrahedron Lett., 2005, 46, 229.
6. Georgin, D.; Taran, F.; Mioskowski, C. Chem. Phys. Lipids., 2003, 125, 83.
7. Aizpurua, JM; Cossio, FP; Palomo, CJ Org. Chem., 1986, 51, 4941.
8. Pandey, MK; Bisai, A.; Singh, VK Tetrahedron Lett., 2004, 45, 9661.
9. Rocca, P.; Marsais, F.; Godard, A.; Queguiner, G.; Adams, L.;Alo, B. Tetrahedron Lett., 1995, 36, 7085.
10. "Modern Organic Synthetic Reagents - Properties, Preparation and Reaction", edited by Hu Yuefei and others
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.