2/10/2022
Amides are widely used in chemical synthesis, especially the preparation of amides by condensation acylation, which is frequently used in laboratory synthesis. The reaction of carboxylic acids and amines is an important method for the synthesis of amides: this reaction is an equilibrium reaction, using an excess of one of the reactants or removing the water formed in the reaction, is conducive to shifting the equilibrium toward the product. This article describes the condensation acylation of several carboxylic acids with amines.
1. First of all
Amides are commonly used in chemical synthesis, especially for preparing amides by condensation acylation, often used in laboratory synthesis. The reaction of carboxylic acids with amines is an important method for synthesizing amides. This is an equilibrium reaction, and the equilibrium can be changed by using an excess of one of the reactants or by removing the water produced in the reaction towards the product. This article describes the condensation acylation of various carboxylic acids and amines.
2. Método sintético de acilación por condensación de amidas.
2.1 Mixed anhydride chloroformate method
The main application is to react carboxylic acid with ethyl chloroformate or isobutyl ester to form mixed anhydride and then react with amine to obtain the corresponding amide. In this reaction, if the A-site of the acid is hindered or has an electron-withdrawing group, it will sometimes stop at the mixed anhydride step. However, heating can promote its reaction, which can also be used to synthesize unsubstituted amides. (figure 1)

figure 1
2.2 Mixed anhydride carbonyldiimidazole method
Use carbonyldiimidazole (CDI) to react with carboxylic acid to obtain acyl imidazoles with higher activity. Many acyl imidazoles have a certain stability and can be separated sometimes. But generally speaking, it does not need to be separated, and the reaction solution directly reacts with the amine to prepare the corresponding amide; the dimethylated trifluoromethane sulfonate (CBMIT) obtained by the reaction of carbonyldiimidazole and methyl triflate is reported in the literature. ) has a better condensation performance. (figure 2)

figure 2
2.3 Mixed anhydride sulfonyl chloride method
Carboxylic acids and sulfonyl chlorides form carboxylic acid-sulfonic acid mixed anhydrides, which react with amines to give the corresponding amides [1]. Commonly used sulfonyl chlorides include methane sulfonyl chloride (MsCl), p-toluenesulfonyl chloride (TsCl), and p-nitrobenzene sulfonyl chloride (NaCl). Due to its electron-withdrawing properties, p-nitrobenzene sulfonyl chloride reacts with acids to generate higher activity. The mixed acid anhydrides, general secondary amines and tertiary amines, and even amines with large steric hindrance can react smoothly. (image 3)

image 3
2.4 Mixed anhydride Boc anhydride method
The corresponding primary amide can be obtained by reacting the mixed anhydride with the acid with Boc anhydride and ammonia. (Figure 4)
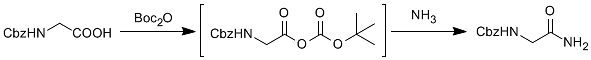
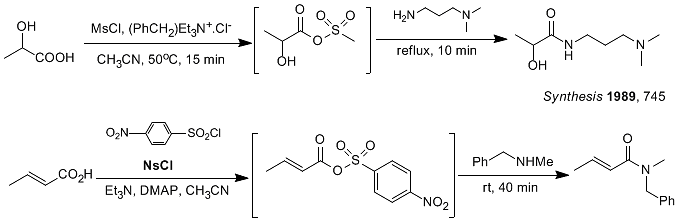
Figure 4
2.5 Carbodiimide condensing agent DCC condensation method
Generally, DCC and DMAP are used together. One of the biggest disadvantages of using DCC is that the other reaction product, dicyclohexylurea, has a small solubility in the general organic phase but is slightly soluble. Therefore, through some commonly used purification methods, such as recrystallization, It is difficult to remove it thoroughly by column chromatography, etc.; since the solubility of dicyclohexylurea in ether is relatively smaller than that of other solvents, it is generally necessary to distill off the reaction solvent and add ether to filter out most of the dicyclohexyl urea. Dicyclohexylurea is then further processed. (Figure 5)
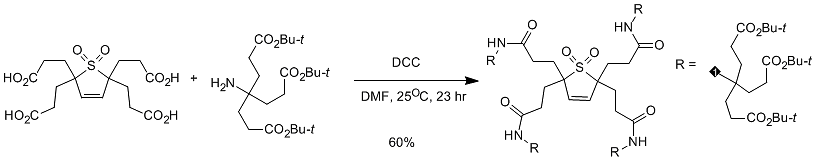
Figure 5
2.6 Carbodiimide condensing agent DIC condensation method
Because the diisopropyl urea produced by DIC has better solubility in common organic solvents, it is generally used more in solid-phase combinatorial chemistry synthesis. (Image 6)
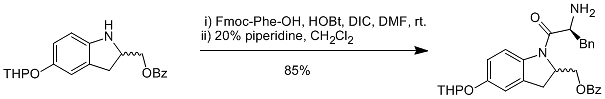
Image 6
2.7 Carbodiimide condensing agent EDCI condensation method
At present, EDCI is the most used in medicinal chemistry. One of its main characteristics is that the urea produced after the reaction is water-soluble and can be easily washed off. Generally, EDCI is used in combination with HOBt (note: this reaction HOBt is generally Indispensable; otherwise, it may cause the condensation yield to be too low). (Figure 7)
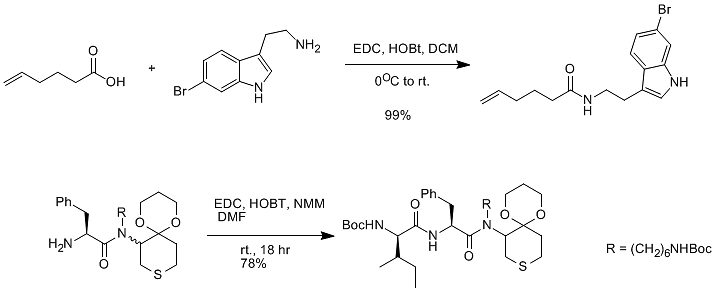
Figure 7
2.8 Condensing agent method of carbenium salts
Currently commonly used O-(7-aza benzotriazole-1-yl)-bis(dimethylamino)carbenium hexafluorophosphate (HATU), O-(benzotriazole-1- Base)-bis(dimethylamino)carbenium hexafluorophosphate (HBTU), O-(5-chlorobenzotriazol-1-yl)-bis(dimethylamino)carbenium hexafluorophosphate (HCTU), O-(benzotriazole-1-yl)-bis(dimethylamino)carbenium tetrafluoroborate (TBTU), O-(N-succinimide)-di (Dimethylamino)carbenium tetrafluoroborate (TSTU), O-(N-endo-5-nor camphene-2,3-carbodiimide)-bis(dimethylamino)carbon Onium tetrafluoroborate (TNTU), etc. (Figure 8)
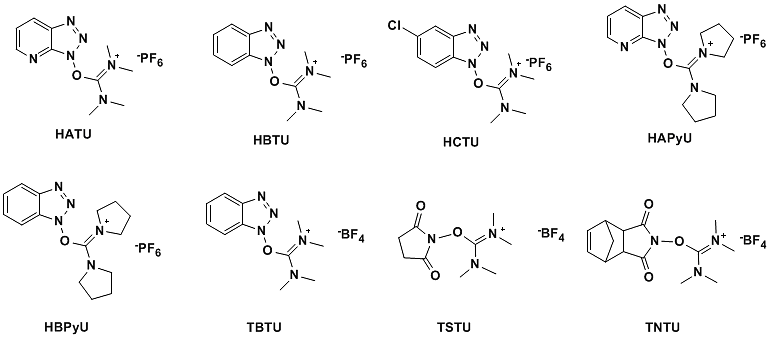
Figure 8
HATU is the most active carbenium salt condensing agent and is often used when other condensing agents do not work well. HBTU can be used in most condensation reactions; however, its low yield is the main reason for its limited use in mass production. HCTU has high activity and can replace HATU for industrial production. Its high activity is due to the more active Cl-HOBt intermediate. TSTU and TNTU can be used for amidation reactions in aqueous solvents. If the dimethylamino group of HATU and HBTU is changed to tetrahydropyrrolyl, O-(7-azabenzotriazol-1-yl)-di(tetrahydropyrrolyl) carbon with higher activity can be obtained Onium hexafluorophosphate (HAPyU), O-(benzotriazol-1-yl)-di(tetrahydropyrrolyl)carbenium hexafluorophosphate (HBPyU).
Using a carbenium salt condensing agent for amide condensation mainly obtains the corresponding active ester in one step through intramolecular transfer. The condensation reaction of HATU is an example of its reaction mechanism. (Figure 9)
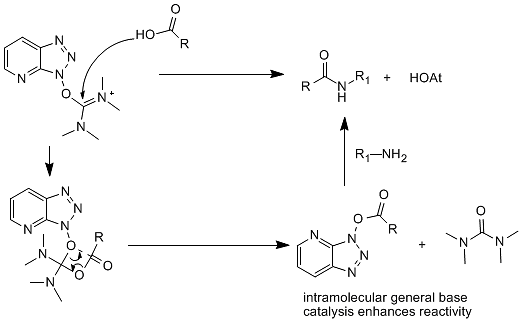
Figure 9
2.9 Condensing agents of phosphonium onium salts
The earliest is the benzotriazol-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate (BOP) reagent, which is a carcinogenic by-product of hexamethylphosphoramide (HMPA). Recently, it has been replaced by the more active benzotriazol-1-yloxy-tris(tetrahydropyrrolyl)phosphonium hexafluorophosphate (PyBOP), which does not produce carcinogenic by-products. (Figure 10)
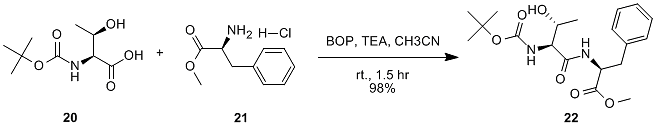
Figure 10
PyBOP is a relatively strong condensing agent. Generally, PyBOP can get better results when other condensing agents do not condense well. For example, PyBOP can condense amino acids with ammonium chloride to obtain the corresponding amino amides. (Figure 11)
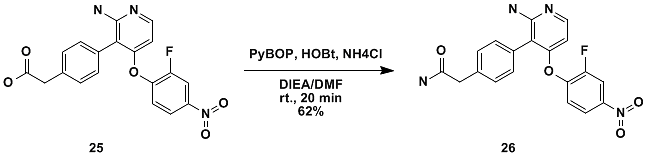
Figure 11
2.10 Organic phosphorus condensation agent
DECP is often used to synthesize a small number of polypeptides, and BOP-Cl is especially suitable for synthesizing amino acids, with good yield and racemization. The disadvantage, however, is that acylated oxazolidines are often obtained when the amine reactivity is low. The poor solubility of BOP-Cl leads to a longer reaction time, sometimes as long as four or five days, and DMF is often used as the reaction solvent. (Figure 12)
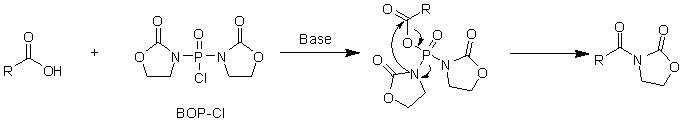
Figure 12
Use DPP-Cl as the condensing agent to synthesize amides [2]: Figure 13 shows only a 15% yield with DCC but a 94% yield with DPP-Cl.
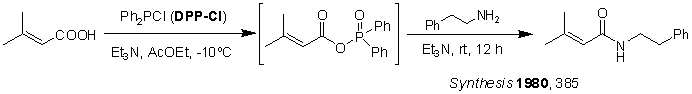
Figure 13
2.11 Other condensing agents
Triphenylphosphine-polyhalomethanes[3], triphenylphosphine-hexachloroacetone[4], triphenylphosphine-NBS[5], etc., can also be used for the condensation of amides. (Figure 14)
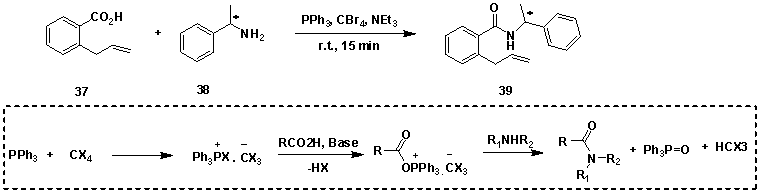
Figure 14
It is reported that DMTMM is used as a condensation agent, and the reaction can be carried out in alcohol or water. (Figure 15)
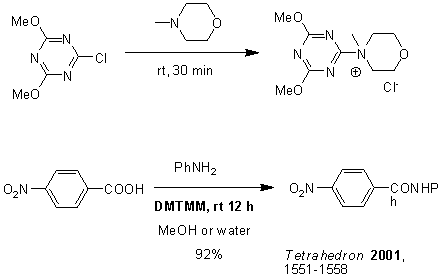
Figure 15
Triphenylphosphine-hexachloroacetone (Fig. 16) and triphenylphosphine-NBS (Fig. 17) can also synthesize amides.
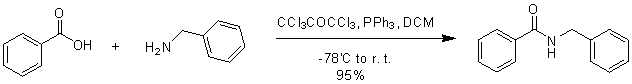
Figure 16
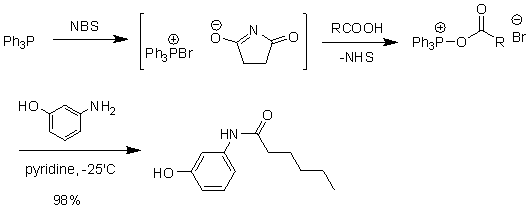
Figure 17
References:
[1] SJ Lau, JP Laussac, B Sarkar. Biochem J. 1989; 257(3): 745-750
[2] Böcker W , Dralle H , Hüsselmann H , Bay V , Brassow M. 1980; 385(2): 187–200.
[3] A Ignatious , J Rahul .1990; 232(2): 1105-1112.
[4] GB Villeneuve, TH Chan.1997; 38: 6489–6492.
[5] Higuchi H, Shimizu K, Ojima J, Sugiura K.1995; 36: 5359-5362.
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.