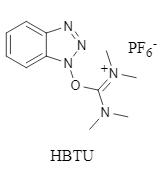
Fig. 1 HBTU structural formula
Applications:
1. HBTU-mediated solid-phase peptide synthesis reactions
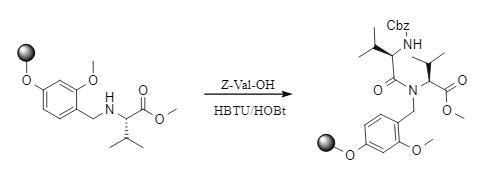
HBTU exhibits excellent site resistance adaptation in solid-phase peptide synthesis and remains highly active even with amine substrates with large spatial site resistance. Gibson's team conducted experiments with a novel linker and carried out a condensation reaction between Z-Val-OH and resin-loaded site-resistant amines. Under alkaline conditions, HBTU synergistically interacted with HOBt to achieve efficient condensation (Fig. 2).
Fig. 2 HBTU-mediated solid-phase peptide synthesis
2. HBTU-promoted synthesis of acyl azides and their derivatives
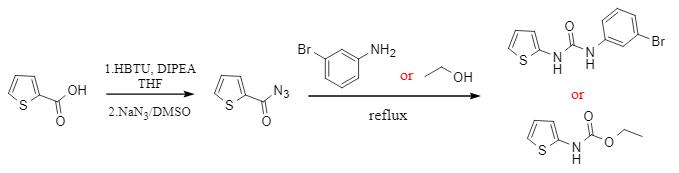
Hemantha's team developed an HBTU-mediated synthesis of carboxylic acids to prepare acyl azides. Under mild alkaline conditions, the efficient, racemization-free conversion of carboxylic acids to acyl azides was achieved by HBTU activation, and the resulting azides could further undergo Curtius rearrangement to generate isocyanates, which could then be reacted with nucleophilic reagents such as alcohols and amines to construct carbamate and urea derivatives (Figure 3) successfully. This tandem reaction can realize the conversion from carboxylic acid to amide derivatives in a one-pot method, which improves the synthetic economy.
Figure 3 HBTU-mediated "one-pot" preparation of urea derivatives and carbamates.
3. Involvement of HBTU in the construction of sulfoguanidines
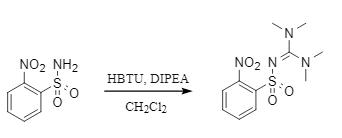
Hénichart's team developed a method for the synthesis of tetramethylsulfoguanidine analogues via HBTU (Fig. 4), in which HBTU and unprotected sulfonamides were synthesized in high yields at room temperature under alkaline conditions. This method has excellent substrate universality and is suitable for both aromatic and aliphatic sulfonamides. It also provides a new synthetic idea for the synthesis of substituted sulfonamides.
Fig. 4 Participation of HBTU in the construction of sulfoguanidine analogs
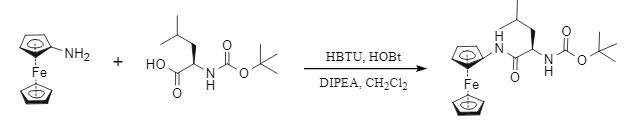
4. Application of HBTU in metal-organic chemistry
In metal-organic chemistry, HBTU still exerts a high coupling ability, and the corresponding coupling products of aminoceramidoferrocene and Boc-glycine derivatives were obtained under the joint action of HBTU and HOBt (Fig. 5).
Fig. 5 HBTU mediates the coupling reaction of metal-organics
5. HBTU as a metal corrosion inhibitor
HBTU is not only an excellent coupling reagent, but also a metal corrosion inhibitor. HBTU significantly reduces metal corrosion by adsorption on the metal surface and larger electron-rich centers. Experimental studies have shown that the inhibition efficiency of HBTU can reach more than 90% in the corrosion system of 0.5 M HCl solution at 303 K. The corrosion inhibition efficiency of HBTU can reach more than 90% in the corrosion system of 0.5 M HCl solution.
In summary, HBTU has a wide range of applications in amide bonding and is an excellent metal corrosion inhibitor with important application value. After 22 years of unremitting efforts and accumulation, Highfine Biotech has continued to plough deep into the field of peptide synthesis reagents worldwide. It has developed into a leading enterprise with extensive customized product coverage capabilities and significant scale production advantages. Now, we can supply diversified specifications of first- to fourth-generation condensation reagents to meet the specific needs of various customers. We sincerely invite customers interested in this product to contact us to learn more about the product and discuss opportunities for cooperation.
References:
[1] Liley, M. J.; Johnson, T.; Gibson, S. E. An Improved Aldehyde Linker for the Solid Phase Synthesis of Hindered Amides[J]. J. Org. Chem., 2006, 71, 1322-1329.
[2] Sureshbabu, V. V.; Lalithamba, H. S.; Hemantha, H. P.; Narendra, N. New and simple synthesis of acid azides, ureas, and carbamates from carboxylic acids: application of peptide coupling agents EDC and HBTU[J]. Org. Biomol. Chem., 2010, 8, 835–840.
[3] Goossens, L.; Hénichart, J. P.; et al. Efficient synthesis of tetramethylsulfonylguanidines between a free sulfonamide group and HBTU[J]. Tetrahedron Lett., 2006, 47, 6087–6090.
[4] Jios, J. L.; Kirin, S. I.; Metzler-Nolte, N.; et al. Synthesis and structural characterization of metallated bioconjugates: C-terminal labeling of amino acids with aminoferrocene[J]. J. Organomet. Chem., 2007, 692, 4209-4214.
[5] Jadhav, S. A. 2-(1H-Benzotriazol-1-yl)-1,1,3,3 tetramethyluronium Hexafluoro phosphate (HBTU)[J]. Synlett, 2010, 8, 1287-1288.
[6] Dahiya, S.; Lata, S.; Yadav, O. S.; Kumar, R. Comparative performance of Uroniums for controlling steel corrosion with methodical inhibition mechanism in acidic medium: Part 1 [J]. J. Mol. Liq., 2016, 221, 124-132.