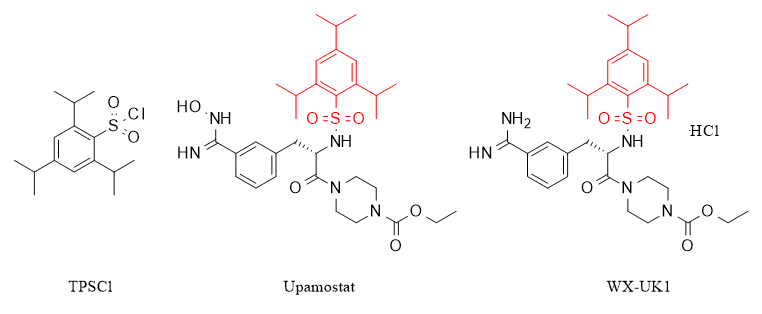
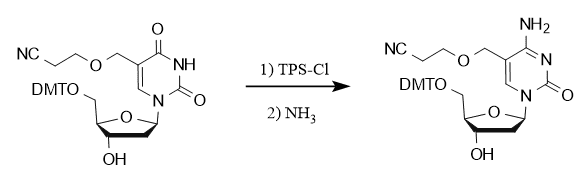2,4,6-Triisopropylbenzenesulfonyl chloride (TPSCl for short), one of the most important organosulfur compounds in organic synthesis, has been widely used in a variety of synthetic applications such as pharmaceuticals, elastomers, dyes, detergents, ion exchange resins, and herbicides. It is worth emphasizing that TPSCl not only exhibits excellent biological activity by itself and is a potent 3MDR protein inhibitor showing good antidepressant activity, but in addition, TPSCl is also the key structural unit of some protease inhibitors, as shown in Fig. TPSCl constitutes a protease inhibitor that exhibits a unique structure with potential functional value.
![TPSCl TPSCl]()
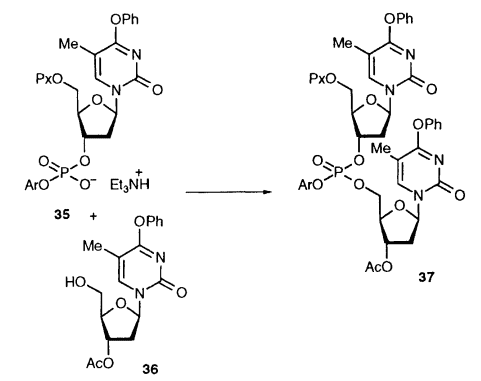
TPSCl is widely used in the field of organic synthesis. It is a highly selective condensing agent, originally proposed by Khorana's team and applied in oligo- and polynucleotide synthesis to construct the bond between C3′-C5′ nucleotides, as shown in the figure below, under the action of TPSCl, the 3′-phosphate group of the first nucleotide unit, and the 3′-phosphate group of the As shown in the figure below, under the action of TPSCl, the 3′-phosphate group of the first nucleotide unit, and the 5′-hydroxyl group of the second nucleotide unit efficiently undergo a condensation reaction. A stable phosphate bond is formed without the need for any additive.
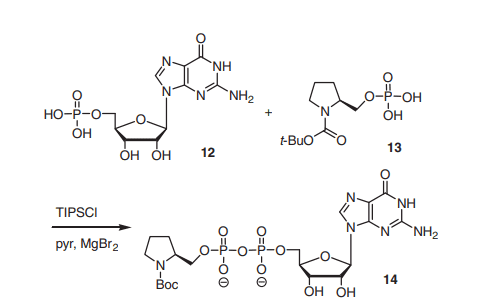
In addition, TPSCl can also be used to synthesize diphosphates, e.g., guanosine-pyrrolidine diphosphate derivatives, which have been suggested as potential fucosyltransferase inhibitors.
TPSCl also plays an important role in the carbonyl amination reaction at the C-4 position of nucleoside bases, as exemplified by the synthesis of 5-hydroxymethyl-2′-deoxycytidine phosphoramidite analogs, in which the conversion of uridine to cytidine was achieved with the participation of TPSCl.
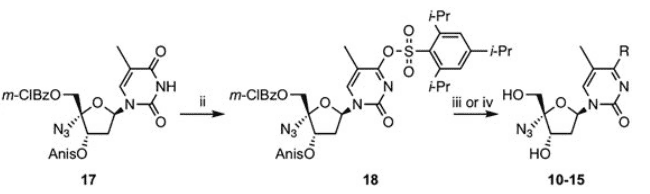
Another example is the synthesis of an active substance against the hepatitis B virus by Imoto's team, which also realized the carbonyl to amino conversion with the help of TPSCl.
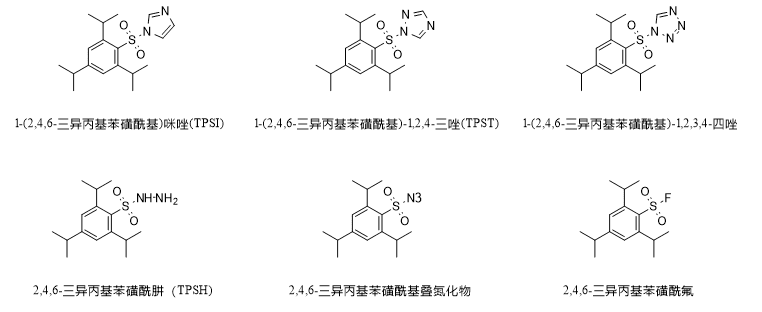
In addition to the above applications, TPSCl is also the main raw material for the synthesis of other multifunctional reagents, as shown in the figure below, including TPSI, TPST, 1-(2,4,6-triisopropylbenzenesulfonyl)-1,2,3,4,-tetrazole, etc., which are used for the condensation of nucleotides; TSFH, which is used for the reduction of olefins and the synthesis of hydrazone; and 2,4,6-triisopropylbenzenesulfonylazide, which is used for the transfer of diazide and azide. isopropylbenzenesulfonyl azide for diazotization and azide transfer.
![TPSCl TPSCl]()
Reference:
[1] Jeelani, A.; Muthu, S.; Irfan, A.; et al. Experimental spectroscopic, molecular structure, electronic solvation, biological prediction and topological analysis of 2, 4, 6-tri (propan-2-yl) benzenesulfonyl chloride: An antidepressant agent[J]. J. Mol. Liq. 2022, 358, 119166.
[2] Renatus, M.; Bode, W.; Huber, R.; et al. Structural and Functional Analyses of Benzamidine-Based Inhibitors in Complex with Trypsin: Implications for the Inhibition of Factor Xa, tPA, and Urokinase[J] J. Med. Chem. 1998, 41, 5445-5456.
[3] Colin, B.R.; Zhang, P.Z. Phosphotriester Approach to the Synthesis of Oligonucleotides: A Reappraisal[J]. J. Chem. Soc., Perkin Trans. 1, 1993, 2291-2301.
[4] Lin, T.C.; Fang, J.M. Diphosphate formation using cyanuric chloride or triisopropylbenzenesulfonyl chloride as the activating agents[J]. Tetrahedron Letters. 2011, 52, 2232-2234.
[5] Zheng Xiu-An. Research on synthetic methods of special nucleosides and nucleoside phosphoryl compounds [D]. Jiangxi: Jiangxi Normal University of Science and Technology, 2019.
[6] Onitsuka, K.; Tokuda, R.; Lmoto, S.; et al. Synthesis and evaluation of the anti-hepatitis B virus activity of 4'-Azido-thymidine analogs and 4'-Azido-2'-deoxy-5-methylcytidine analogs: structural insights for the development of a novel anti-HBV agent[J]. NUCLEOS NUCLEOT NUCL. 2020, 39, 518-529.
[7] Narang, S.A.; Itakura, K.; Katagiri, N. Novel Condensing Reagents for Polynucleotide Synthesis[J]. J.C.S. CHEM. COMM. 1974, 325-326.