Background Information
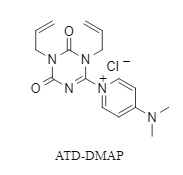
In light of this, the Kunishima team developed a novel condensation reagent, ATD-DMAP (structure shown in Figure 1), containing a DMAP structure. This reagent exhibits excellent stability, remaining stable for more than 6 months. It generates DMAP in situ and uses it as a leaving group in the condensation reagent, which can efficiently promote the esterification of carboxylic acids and alcohols and the amidation of carboxylic acids and amines in a short time, yielding the target compound in high yield.
Figure 1. Structure of ATD-DMAP
ATD-DMAP Applications:
ATD-DMAP, a novel, multifunctional, and highly efficient condensation reagent, not only promotes esterification and amidation reactions but also exhibits strong racemic inhibition capabilities.
1. Esterification reaction
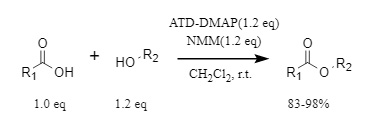
Using dichloromethane as the reaction medium and N-methylmorpholine (NMM) as the base, ATD-DMAP can efficiently promote esterification reactions within 10 minutes at room temperature, with yields reaching up to 98%. This reagent is suitable for combinations of various carboxylic acids and alcohols, demonstrating good substrate applicability (Figure 2).
Figure 2. ATD-DMAP used in esterification reaction
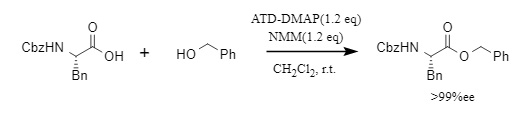
For chiral substrates, ATD-DMAP can quantitatively generate the corresponding chiral products, exhibiting excellent racemic inhibition ability (Figure 3).
Figure 3. ATD-DMAP used for the esterification of chiral substrates.
Furthermore, this reagent exhibits excellent compatibility with bases and solvents: it works better with organic bases than with inorganic bases, achieving high yields with no significant loss in common solvents such as acetonitrile, tetrahydrofuran, and ethyl acetate.
2. Amide Reaction
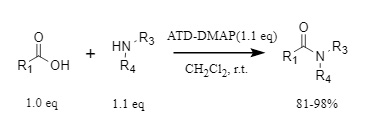
ATD-DMAP is also suitable for the amidation reactions of carboxylic acids and amines, converting aromatic, aliphatic, and α,β-unsaturated carboxylic acids into the corresponding amide products with excellent yields (Figure 4).
Figure 4. ATD-DMAP used in amidation reaction
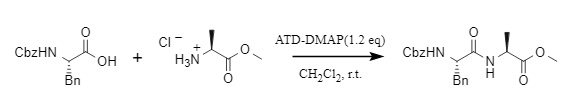
Furthermore, ATD-DMAP can also be used to synthesize peptides with high yields, and NMR spectroscopy shows no racemization products (Figure 5), further confirming its ability to effectively inhibit the formation of racemization products.
Figure 5. ATD-DMAP for peptide synthesis
Although ATD-DMAP exhibits high efficiency in most cases, its efficiency is lower for reactions involving certain sterically hindered carboxylic acids.
3. Reaction Mechanism
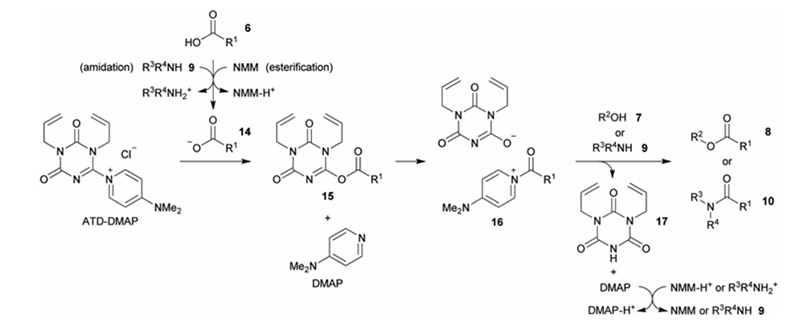
The possible mechanism of ATD-DMAP is shown in Figure 6: The carboxylate attacks the triazinone moiety in ATD-DMAP to form an activated ester, which then releases free DMAP to react with the activated ester, yielding an N-acylpyridine intermediate. Further alcoholysis or ammonolysis reactions occur to yield the corresponding ester and amide.
Figure 6. Mechanism of ATD-DMAP
Overall conclusion:
The novel condensation reagent ATD-DMAP developed by the Kunishima team possesses advantages such as good stability, ease of operation, rapid reaction, and high yield. This reagent can promote the esterification of carboxylic acids with alcohols and the amidation of carboxylic acids with amines, making it suitable for the rapid synthesis of a variety of substrates. Furthermore, ATD-DMAP exhibits excellent racemization inhibition capabilities in the synthesis of esters or amides with chiral centers, effectively avoiding the generation of racemization byproducts. Therefore, this reagent has broad application prospects in fields such as pharmaceutical synthesis.
About Highfine Biotech:
Suzhou Haofan Biotech Co., Ltd. (Stock Code: 301393.SZ), founded in 2003 and headquartered in Suzhou High-tech Zone, is a national high-tech enterprise providing specialty raw materials to global pharmaceutical R&D and manufacturing companies. Its products are mainly used in peptide, nucleotide, and pharmaceutical synthesis, covering a wide range of products including condensing agents for specialty amide bond formation, protective agents, linking agents, protein cross-linking agents for antibody-drug conjugates, molecular building blocks, liposomes, and phosphorus reagents. Currently, it has developed and produced over 1500 products.
After 22 years of unremitting efforts and accumulation, Haofan Biotech has continuously deepened its expertise in the global peptide synthesis reagent field and has now developed into a leading enterprise with extensive customized product coverage and significant advantages in large-scale production, capable of meeting the specific needs of various customers. We sincerely invite customers interested in our products to contact us for further information and to discuss cooperation opportunities.
References:
[1] Liu, J.; Fujita, H.; Kunishima, M., et al. Development of a triazinedione-based dehydrative condensing reagent containing 4-(dimethylamino) pyridine as an acyl transfer catalyst[J]. Org. Biomol. Chem., 2021, 19, 4712−4719.