5/21/2022
2-(Trimethylsilyl) methoxymethyl chloride, referred to as SEM-Cl, is a commonly used protective reagent, the English name is 2-(Trimethylsilyl) methoxymethyl chloride, the molecular formula is C6H15ClOSi, the structural formula is shown in Figure 1, and the molecular weight It is 166.72, and its CAS number is 76513-69-4. It is a colorless and transparent liquid at room temperature, soluble in various organic solvents such as dichloromethane, ether, tetrahydrofuran, etc., and reacts slowly with water, so it needs to be protected by an inert gas. Store dry.
2-(Trimethylsilyl) methoxymethyl chloride, referred to as SEM-Cl, is a commonly used protective reagent, the English name is 2-(Trimethylsilyl) methoxymethyl chloride, the molecular formula is C6H15ClOSi, the structural formula is shown in Figure 1, and the molecular weight It is 166.72, and its CAS number is 76513-69-4. It is a colorless and transparent liquid at room temperature, soluble in various organic solvents such as dichloromethane, ether, tetrahydrofuran, etc., and reacts slowly with water, so it needs to be protected by an inert gas. Store dry.

Formula 1
SEM-Cl has the following common functions in daily organic synthesis reactions.
01 As a hydroxyl-protecting group reagent
SEM-Cl is now widely used in organic synthesis as a hydroxyl-protecting reagent, which can be used to protect both primary alcohols (Equation 2) and secondary and tertiary alcohols (Equations 3, 4).

Formula 2

Formula 3

Formula 4
The SEM protecting group is stable under acidic conditions that hydrolyze THP, TBDMS, and MOM groups, but will be removed under strongly acidic conditions such as trifluoroacetic acid. When SEM is used as a phenolic protecting group, it can be removed with phosphorus tetraiodide (Equation 5).
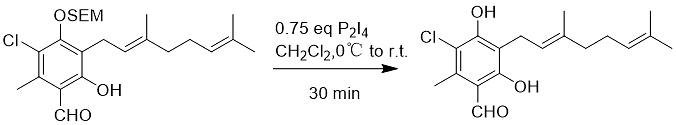
Formula 5
SEM-Cl can be used to protect carboxylic acids (Equation 6).
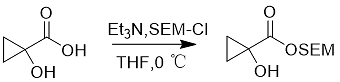
Formula 6
Under the action of methanol reflux or magnesium bromide, the SEM group protecting the carboxyl group can be removed (Eq. 7).
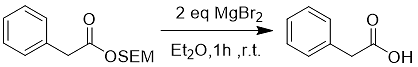
Formula 7
SEM-Cl can be used to protect secondary aromatic amines and is an ideal reagent for protecting imidazoles, indoles, and pyrroles (Equation 8).
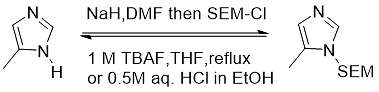
Formula 8
When many functional groups are protected by the SEM group, the stability is very good when the molecule is further functionalized. In recent years, a one-pot synthetic route to protect and alkylate imidazoles using butyllithium and SEM-Cl has been developed (Eq. 9).
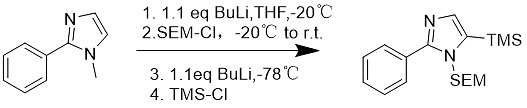
Formula 9
SEM-Cl can be used to replace formaldehyde (Eq. 10).
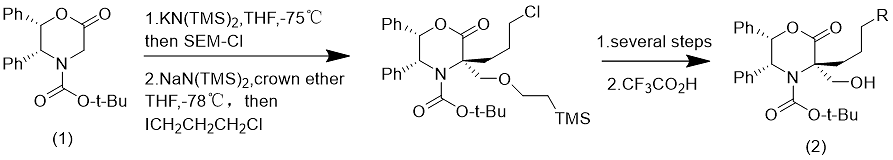
Formula 10
Compound (1) is alkylated by SEM-Cl, and the resulting SEM substituent can be subsequently removed with trifluoroacetic acid to obtain compound (2).
SEM-Cl has been used as a formaldehyde substitute in some synthetic fields (Eq. 11).
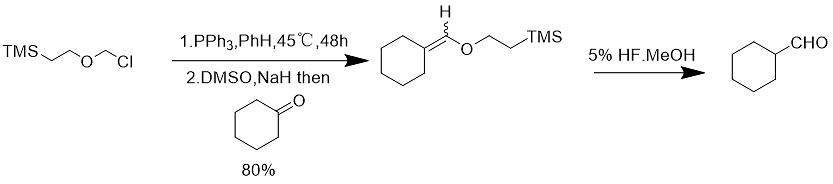
Formula 11
SEM-Cl was converted to onium ylide after treatment with triphenylphosphine and sodium hydride, and this onium ylide reacted with various aldehydes and ketones to form enol ethers, which were then hydrolyzed with 5% hydrofluoric acid to obtain the corresponding aldehydes.
This article briefly introduces some applications of SEM-Cl in organic synthesis. It is widely used and has the characteristics of mild reaction conditions, specific product selectivity, and high yield.
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.