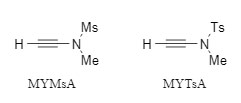1. Ynamide (Zhao reagent)
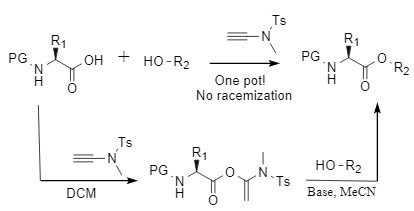
Alkynamide condensation reagents are a type of non-racemization condensation reagent developed by Professor Zhao Junfeng, collectively known as Zhao reagents (representative products are shown in Figure 1). The condensation reaction in which they participate does not require the addition of additional catalysts, and they show good tolerance to air and water. The target amide is obtained under near-neutral conditions, thus avoiding the racemization problem caused by alkali. Zhao reagents have been successfully used in the construction of active molecules such as amides, macrolides, and thioamides through the "two-step one-pot" synthesis strategy (Figure 2). For more information, please refer to "A new type of highly efficient, non-racemization alkynamide condensation reagent"
Figure 1: Zhao reagent
Figure 2: Condensation reaction mediated by the Zhao reagent
2. Allenone
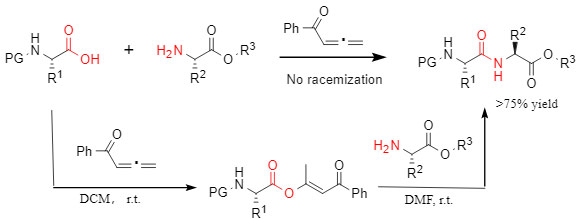
Professor Zhao Junfeng's research group has developed a ketone-mediated amide bonding method, which provides a new solution for the construction of amide bonds. This method does not require the participation of additional catalysts and additives. Its reaction pathway is to generate an ester intermediate through the reaction of carboxylic acid and ketone, and then react with amine to obtain the corresponding amide product, avoiding the risk of racemization and reducing the occurrence of other side reactions. At the same time, it shows a wide range of adaptability to a variety of natural amino acids and non-natural amino acids. (Figure 3)
Figure 3: Phenyl allenone-mediated condensation reaction
3. Oxyma Series
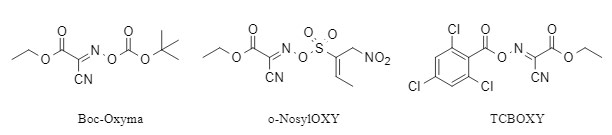
Since Oxyma was proven to have a breakthrough advantage over traditional benzotriazole reagents in condensation reactions, it quickly attracted widespread attention from scientists, and a series of Oxyma derivatives were developed, which have the characteristics of high efficiency, no racemization, and no explosion risk. The following figure shows several representative Oxyma series derivatives that do not produce racemization (see Figure 4). The Oxyma by-products after the reaction can be recycled and regenerated into corresponding derivatives, minimizing the amount of chemical waste. At present, the Oxyma series derivatives have been successfully used in the construction of key structural units such as amides and esters. For a detailed introduction, please refer to "Boc-Oxyma: 'One agent, two effects" to build a new green synthesis system.
Figure 4 Oxyma derivatives
4. TCFH-NMI
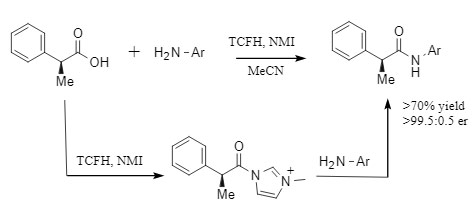
The combination of N,N,N',N'-tetramethylchloromethylammonium hexafluorophosphate (TCFH) and N-methylimidazole (NMI) is a highly efficient amide condensation system developed by Bristol-Myers Squibb. This golden combination promotes the condensation reaction by in situ generating highly reactive N-acyl imidazolium salts (see Figure 5), showing technical advantages such as a wide range of applicable substrates and no diastereoisomerization, and is particularly suitable for condensation reactions involving low-activity amines and large sterically hindered carboxylic acids. For more information, please refer to "The Golden Combination for Efficient Acid-Amine Condensation: TCFH-NMI."
Figure 5 TCFH-NMI-mediated condensation reaction
5. Xiangshan Reagent
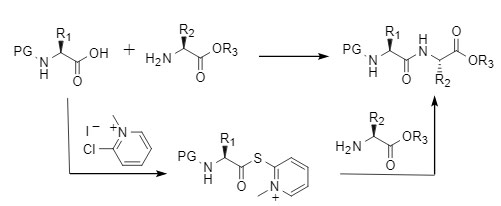
2-Chloro-1-methylpyridinium iodide, referred to as CMPI, is also known as Mukaiyama reagents. It is easy to operate and simple to post-process. It is a condensation reagent with commercial value. The condensation reaction involving CMPI is generally carried out under weak alkaline conditions. It activates carboxylic acids to form carboxylic acid esters, and then undergoes aminolysis and alcoholysis reactions through nucleophilic attack, efficiently constructing key structural units, and providing excellent functional group compatibility and racemization inhibition capabilities (Figure 6).
Figure 6: Condensation reaction mediated by Mukaiyama reagent
6. NDTP
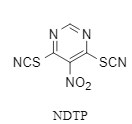
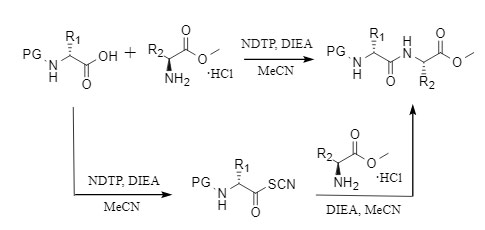
5-Nitro-4,6-dithiocyanatopyrimidine (NDTP, Figure 7) developed by the research group of Academician Wang Rui is a new condensation reagent that is mild, readily available and recyclable. NDTP promotes carboxylic acids to form acyl thiocyanate intermediates, which have high stability and acyl chloride-like activity, and are then attacked by amine/alcohol reagents, which can form bonds quickly without racemization (Figure 8). It is worth noting that in the NDTP-mediated esterification reaction, esters can be formed in 1 minute under mild conditions.
Figure: NDTP structure
Figure 8 :NDTP-mediated condensation reaction
7. T3P
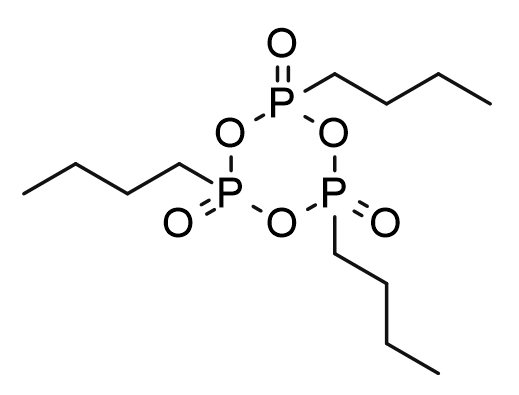
A mild combination of n-propylphosphonic anhydride (T3P) and pyridine was developed to construct amide bonds. This system can promote the coupling reaction of a variety of easily racemic substrates and provide amides with high yields and high enantiopurity. Currently, T3P is listed as a monitored chemical and is restricted in transportation, use, storage, etc. As an alternative, n-butylphosphonic anhydride (T4P) can provide equal or even higher reaction efficiency and stereocontrol capabilities (Figure 9).
Figure 9: Structural formula of T3P and T4P
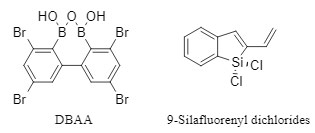
8. Other reagents
In addition to the above-mentioned mainstream reagents, a series of substances such as silanes and boric anhydride are also gradually being used as condensation reagents, which are characterized by their high activation ability, wide substrate applicability, and excellent racemization inhibition ability.
After 22 years of unremitting efforts and accumulation, Haofan Biotech has continued to deepen its presence in the field of global peptide synthesis reagents and has now developed into a leading company with extensive customized product coverage capabilities and significant scale production advantages. It can now supply racemization inhibition reagents of various specifications to meet the specific needs of various customers. We sincerely invite customers who are interested in this product to contact us to learn more about the product details and explore cooperation opportunities.
References:
[1] Duengo, S.; Muhajir, M. I.; Maharani, R.; et al. Epimerisation in Peptide Synthesis[J]. Molecules, 2023, 28, 8017.
[2] Guo, Y. Y.; Wang, M. Y.; Gao, Y.; Liu, G. D. Recent advances in asymmetric synthesis of chiral amides and peptides: racemization-free coupling reagents[J]. Org. Biomol. Chem., 2024, 22, 4420-4435.
[3] Wang, X.W.; Yang, Y.; Zhao, J.F.; et al. Ynamide-Mediated Intermolecular Esterification[J]. J. Org. Chem. 2020, 85, 6188-6194.
[4] Wang, Z.N.; Wang, X.W.; Wang, P.H.; Zhao, J.F. Allenone-Mediated Racemization/Epimerization-Free Peptide Bond Formation and Its Application in Peptide Synthesis[J]. J. Am. Chem. Soc., 2021, 143, 10374-10381.
[5] Beutner, G.L.; Young, I.S.; Ye, Q.M.; et al. TCFH−NMI: Direct Access to N‑Acyl Imidazoliums for Challenging Amide Bond Formations[J]. Org. Lett. 2018, 20, 4218-4222.
[6] Li, Y.P.; Wang, P.; Wang, R.; et al. NDTP Mediated Direct Rapid Amide and Peptide Synthesis without Epimerization[J]. Org. Lett., 2022, 24, 1169-1174.