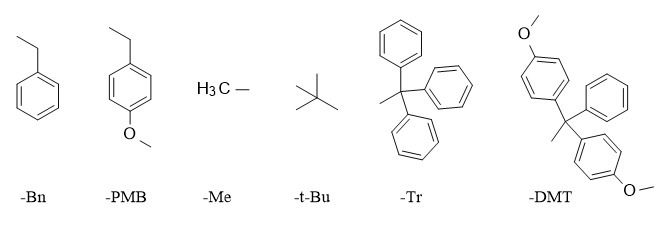
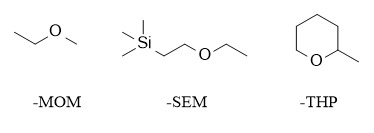
Since there are many types of functional groups and more methods for protecting groups, this article mainly introduces the methods for protecting and deprotecting alcoholic hydroxyl groups.
Hydroxyl groups are widely present in many compounds with physiological significance and synthetic value, such as nucleosides, carbohydrates, steroids, macrolide compounds, etc. In addition, hydroxyl groups are also very important functional groups in organic synthesis, which can be converted into carboxyl groups, carbonyl groups, halogens, and other functional groups. However, hydroxyl groups are also prone to oxidation, acylation, substitution, and other reactions. Therefore, in organic synthesis, especially in the process of converting many functional groups, we need to protect hydroxyl groups.
There are many protecting groups for hydroxyl groups, but only a few of them are widely used in practice. The protection of hydroxyl groups is mainly achieved by converting them into ethers or esters. Below we will focus on the application of ether-protecting groups in hydroxyl protection.
Compared with ester-protecting groups, ether-protecting groups are more widely used in hydroxyl protection, mainly including silyl ethers, alkyl ethers, alkoxy alkyl ethers, etc. Among them, silyl ether-protecting groups are favored because of their easy introduction and removal and are one of the most common methods for protecting hydroxyl groups.
1. Alkyl ethers
The commonly used protecting groups of alkyl ethers include benzyl (Bn), p-methoxybenzyl (PMB), trityl (Tr, DMT), methyl (Me), tert-butyl (t-Bu), etc.
(1) Benzyl:
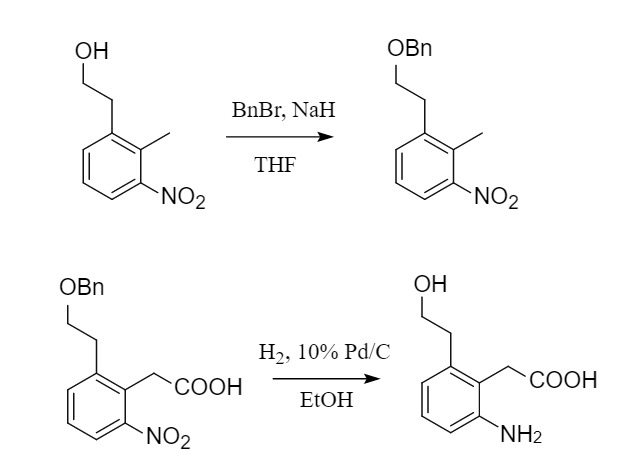
Benzyl is a commonly used ether-protecting group, usually introduced by Williamson synthesis, obtained by reacting halogenated hydrocarbons with the corresponding sodium alcohol in DMF or THF. In actual synthesis, according to the acidity of different hydroxyl groups, a suitable base is selected to react with BnCl or BnBr.
The removal of benzyl is usually removed by hydrogenolysis. Pd/C is the most commonly used catalyst. The hydrogen source can be hydrogen gas, cyclohexene, ammonium formate, isopropanol, etc. In addition, benzyl can also be removed under acidic conditions such as Lewis acid TMSI, FeCl3, etc.
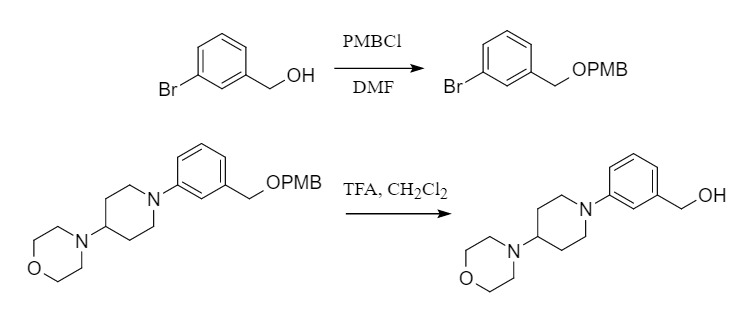
(2) p-Methoxybenzyl:
The introduction and removal methods are similar to those of benzyl. PMB-protecting groups are often removed in trifluoroacetic acid (TFA) (such as TFA, CH2Cl2, r.t.). In addition, PMB is also commonly removed by oxidation, such as DDQ/CH2Cl2-H2O, and CAN/MeCN. Therefore, p-methoxybenzyl can be selectively removed in the presence of benzyl.
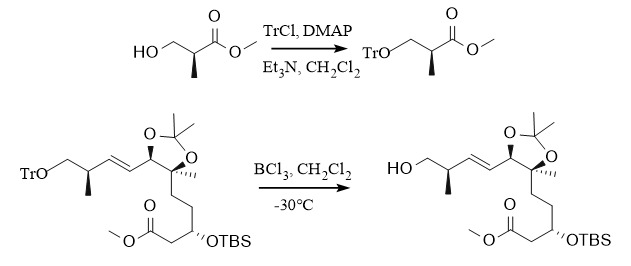
(3) Trityl protecting groups (Tr, DMT)
Common trityl-protecting groups include triphenyl methyl (Tr) and 4,4'-dimethoxy trityl (DMT). Due to their steric hindrance, they are usually used to protect primary alcohols. Among them, the introduction of trityl ether is commonly used in the following systems: ①: Tr-Cl, DMAP, Py; ②: Tr-Cl/Et3N/DMAP, CH2Cl2, etc. The removal method generally uses acidic conditions: such as ①: TsOH,/MeOH, ②: AcOH, and can also be removed by Pd/C hydrogenation. 4,4'-Dimethoxytrityl is also often used to protect hydroxyl groups, and the introduction and removal methods are similar to Tr.
(4) Methyl:
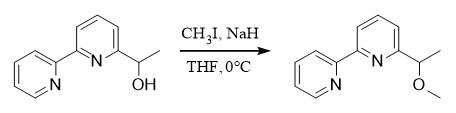
Methyl ether is mainly introduced through reagents such as dimethyl sulfate, methyl iodide, and diazomethane. Common systems include ①: NaH/Me2SO4; ②: NaH/MeI, etc. Methyl ether is relatively stable and difficult to remove. It is usually removed by the BBr3/CH2Cl2 system. It can also be removed by TMSI/ CH2Cl2 or the MeCN system. Compared with alcohol hydroxyl groups, phenolic hydroxyl groups are often protected by methyl ethers and are easier to remove.
2. Silyl ether
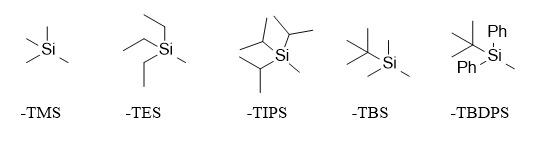
Silyl ether-protecting groups are widely used in the protection of hydroxyl groups. Different substituents connected to the silicon atom make their stability and activity different. Commonly used silyl ether protecting groups include trimethylsilyl (TMS), triethylsilyl (TES), triisopropylsilyl (TIPS), tert-butyldimethylsilyl (TBS), and tert-butyldiphenylsilyl (TBDPS). In addition, different silyl ether-protecting groups have different steric hindrances. Based on this characteristic, hydroxyl groups can be selectively protected. For example, TBDPS, a protecting group with a large steric hindrance, cannot be used to protect tertiary alcohols; TMS, a protecting group with a small steric hindrance, can protect primary alcohols, secondary alcohols, and tertiary alcohols with a small steric hindrance. In addition, the source of the protecting group will also have an impact. For example, TMSOTf and TBSOTf, which are more active, can be used to protect secondary alcohols or tertiary alcohols with large steric hindrance.
![highfine highfine]()
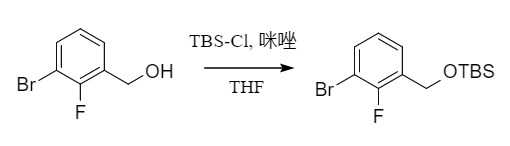
Silyl ether protection is usually carried out by reacting the corresponding chlorosilane with alcohol under alkaline conditions, such as TBS-Cl/imidazole.
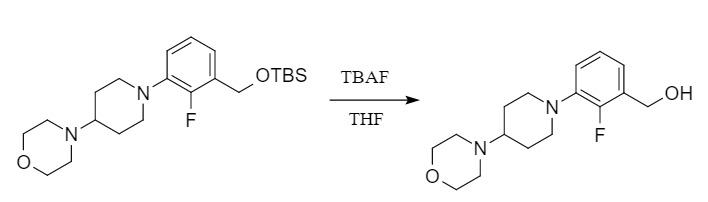
The removal of silyl ether-protecting groups can be carried out under acidic or alkaline conditions. Different alkyl silyl ethers have different sensitivity to acid or base, and the deprotection conditions are also different. The relative stability of several common silyl ether protecting groups to acid hydrolysis is in the following order: TMS < TES < TBDMS < TIPS < TBDPS; the relative stability to the base is in the following order: TMS < TES < TBDMS ≈ TBDPS < TIPS. In addition, due to the strong affinity between fluorine and silicon atoms, fluoride anion (F-) becomes the most commonly used deprotection method. The related deprotection reagents include tetraalkylammonium fluorides, such as TBAF (tetrabutylammonium fluoride)/THF, pyridine hydrochloride, and HF/MeCN.
3. Alkoxyalkyl ethers
Alkoxyalkyl ether protecting groups can also be called acetal protecting groups, a more commonly used type of protecting group. Commonly used protecting groups include methoxymethyl (MOM), trimethylsilylethoxymethyl (SEM), and 2-tetrahydropyranyl (THP). These protecting groups are acid-sensitive and are usually removed under acidic conditions.
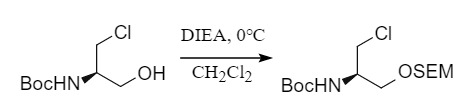
Among them, the introduction of MOM and SEM protecting groups is mostly carried out under alkaline conditions, using the corresponding chlorinated compounds to react with alcohols. Commonly used systems include NaH/THF, DIEA/CH2Cl2, etc. Deprotection is carried out under acidic conditions. MOM requires stronger acidity, while SEM is more sensitive to acid and can be removed under weakly acidic conditions. In addition, the SEM protecting group can also be removed by TBAF because it contains a silicon group.
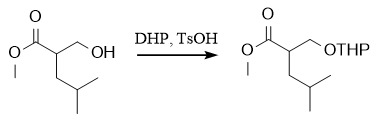
The THP protecting group is relatively special. It will introduce a new chiral center after protection. Generally, the introduction of THP is carried out under acid catalysis, such as pyridine p-toluenesulfonate (PPTS)/DHP/CH2Cl2; and TsOH/DHP/CH2Cl2. THP ether is relatively stable under strongly acidic conditions and can be removed under milder conditions such as HOAc/THF/H2O (4:2:1)/45°C.
This article mainly introduces the application of ether-protecting groups in hydroxyl protection, aiming to broaden the range of choices for researchers in synthetic work. In actual operation, researchers can choose appropriate protection and deprotection strategies according to specific experimental conditions to achieve higher synthesis efficiency.
Since its establishment in 2003, Haofan Biotech Co., Ltd. has focused on the research, development, and sales of condensation agents, protective reagents, peptide drugs, and liposome membrane materials. The company has now launched a variety of protective reagents, such as Fmoc-Cl/Fmoc-Osu, Troc-Cl/Troc-Osu, Tr-Cl, DMT-Cl, etc. We look forward to interested customers calling us for consultation and discussing cooperation opportunities.