1. Ester protecting groups (acyl protecting groups)
Ester protecting groups are an economical and effective method for protecting hydroxyl groups. They can be roughly divided into carboxylic acid esters, carbonates, and sulfonic acid esters, among which carboxylic acid ester-protecting groups are the most commonly used.
1. Carboxylic acid esters
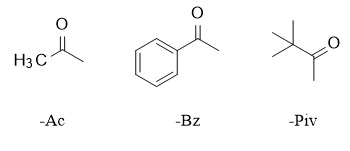
Common carboxylic acid ester protecting groups include acetyl (Ac), benzoyl (Bz), pivaloyl (Piv), etc., which are widely used in the synthesis of sugar chemistry, peptide chemistry, etc. The methods for introducing acyl groups are generally similar. The corresponding acyl chloride or acid anhydride reacts with alcohol in the presence of a base (mainly pyridine and triethylamine) to obtain the corresponding ester. If the reaction is too slow, especially for substrates with large steric hindrance, DMAP can be added to accelerate the reaction. In addition, in polyhydroxy substrates, pivaloyl chloride can selectively protect primary alcohols.
![highfine highfine]()
In general, ester groups can be removed by hydrolysis under alkaline conditions, such as K2CO3, NH3, hydrazine hydrate, etc. The order of the hydrolysis ability of the acyl groups mentioned above is roughly: pivaloyl Piv<benzoyl Bz<acetyl Ac. By utilizing the difficulty of hydrolysis of these protecting groups, the acetyl group can be selectively removed in the presence of pivaloyl. Common conditions and examples for introduction and removal are as follows:
(1) Acetyl
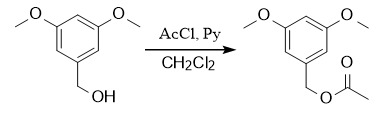
Protection: ①: Ac2O or AcCl, Py, DMAP; ②: Ac2O, TMSOTf (Cat.), CH2Cl2, 0℃;
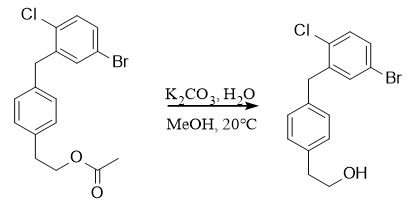
Deprotection: ①: K2CO3, MeOH/H2O, 20℃; ②: NH2NH2, MeOH
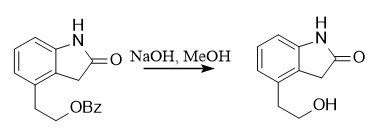
(2) Benzoyl
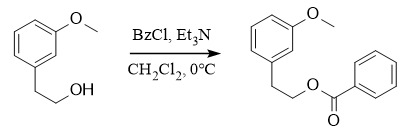
Protection: BzCl, Et3N, CH2Cl2, 0℃
Deprotection: ①: NaOH, MeOH; ②: Et3N, MeOH, H2O (1:5:1), reflux
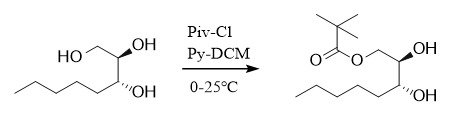
(3) Pivaloyl
Protection: ①: PivCl, Py, 0-25℃, DCM; ②: Piv2O, MgBr2, TEA, CH2Cl2, r.t.;
Deprotection: ①: Bu4N+OH-, 20℃; ②: MeONa, MeOH
2. Carbonate
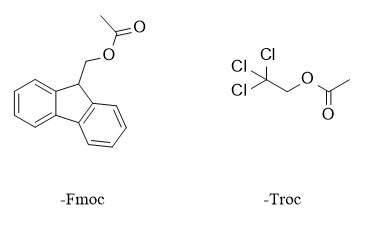
Carbonate is also a means of protecting hydroxyl groups. It can be removed by alkaline hydrolysis or by using a second alkyl substituent. Commonly used protecting groups include 9-fluorenyl methoxycarbonyl (-Fmoc), 2,2,2-trichloroethoxycarbonyl (-Troc), etc. The common conditions and examples for introduction and removal are as follows:
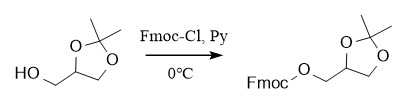
(1) 9-fluorenyl methoxycarbonyl:
Protection: Fmoc-Cl, Py, 0℃; Deprotection: ①: Et3N, Py; ②: DBU, CH2Cl2
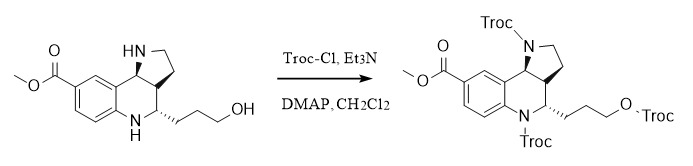
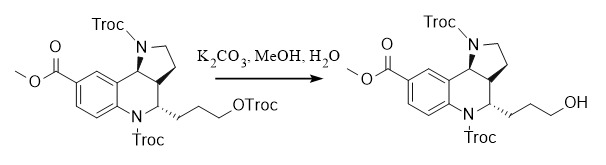
(2) 2,2,2-trichloroethoxycarbonyl:
Protection: ①: Troc-Cl, Py, 20℃; ②: Troc-Cl, Et3N, DMAP, CH2Cl2
Deprotection: ①: Zn/AcOH, 20℃; ②: K2CO3, MeOH/H2O
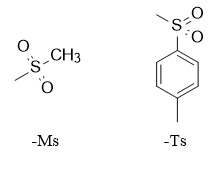
3. Sulfonate
Sulfonate protecting groups are mainly used to protect sugar compounds, and can also be used in certain specific compounds. Commonly used ones include methanesulfonyl (Ms), p-toluenesulfonyl (Ts), etc. However, in many cases, the introduction of sulfonyl (OMs, OTs) is more likely to react as a leaving group.
(1) Methanesulfonyl:
Protection: Ms-Cl, Et3N, CH2Cl2, 0℃; Deprotection: Na(Hg), i-PrOH
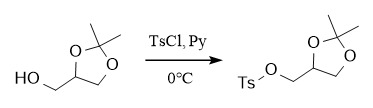
(2) p-Toluenesulfonyl
Protection: Ts-Cl, Py, CH2Cl2, 0℃; Deprotection: hv, Et3N, MeOH
2. Protection of diols
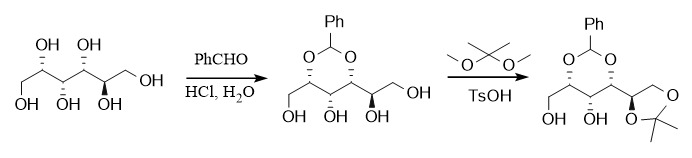
Diols (1,2-diols and 1,3-diols) are very common in synthetic design and natural compounds such as macrolides, and many corresponding protecting groups have been derived. The protection of diols is usually achieved by using aldehydes and ketones. Similarly, aldehydes and ketones can also be protected by diols. The common protecting groups are called acetals and ketals. Among them, dioxolane and dioxane are the most common protecting groups for diols. Based on the factors of thermodynamic stability, acetal-protecting groups tend to form a six-membered ring, namely dioxane, while ketal-protecting groups are more likely to form a five-membered ring. For example, the most commonly used acetal is the benzylidene protecting group, and the introduction methods include PhCHO/ZnCl2 or H+, PhCH(OMe)2/TsOH/DMF, etc.; the most commonly used ketal is the acetone acetal protecting group, which is introduced by acid catalysis using acetone or 2,2-dimethoxypropane.
Acetals and ketals are generally acid-sensitive and can be deprotected under acidic conditions. In addition, the benzylidene-protecting group can also be removed by hydrogenolysis.
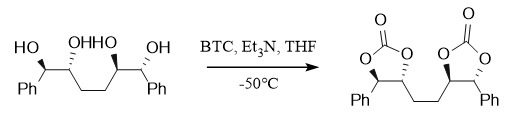
In addition to acetals and ketals, carbonates can also be used to protect diols. For example, triphosgene reacts with 1,2-diols in the presence of a base (Py, Et3N) to form carbonates, such as:
This article mainly introduces the application of ester-protecting groups in hydroxyl protection and the protection and deprotection of diols, aiming to broaden the range of choices for researchers in synthetic work. In actual operation, researchers can choose appropriate protection and deprotection strategies according to specific experimental conditions to achieve higher synthesis efficiency.
Since its establishment in 2003, Haofan Bio has focused on the research, development, and sales of condensation agents, protective agents, peptide drugs, and liposome membrane materials. The company has now launched a variety of protective reagents, such as Fmoc-Cl/Fmoc-Osu, Troc-Cl/Troc-Osu, Tr-Cl, DMT-Cl, etc. We look forward to interested customers calling us for consultation and discussing cooperation opportunities.