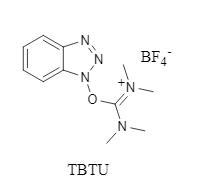
Figure 1: TBTU structure
Application:
1. TBTU-mediated amide bonding reaction
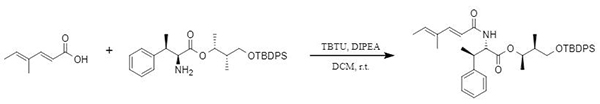
TBTU is an efficient amidation reagent with high racemization inhibition, widely used in peptide synthesis and the synthesis of drugs and other active substances. The Dumpala team developed a synthesis method for Jomthonic Acid A1, using TBTU to achieve efficient bonding in the coupling reaction of key fragments (Figure 2). In addition, TBTU has high stereoselectivity in the coupling reaction. In a weakly alkaline environment, the TBTU-mediated reaction of N-acetyl-L-phenylalanine and aniline has excellent stereochemistry (Figure 3). Also, it provides a new synthetic idea for the synthesis of acetylated protected amino acids.
Figure 2: TBTU-mediated synthesis of Jomthonic Acid A1 fragment
Figure 3: TBTU-mediated coupling reaction of N-acetyl-L-phenylalanine
2. TBTU-promoted esterification reaction and selective esterification synthesis strategy
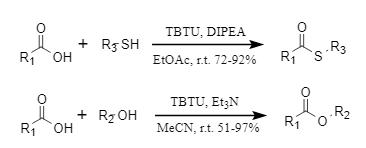
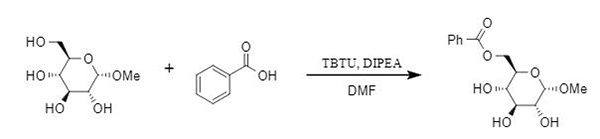
Traditional esterification methods struggle to overcome the problems of large steric hindrance and acid-base sensitivity in substrates, and promoting esterification with condensation reagents is an effective solution. TBTU can achieve efficient esterification of alcohols, phenols, and thiols at room temperature (Figure 4). It is worth noting that the Grindley team found that TBTU has a high selectivity for primary alcohols when promoting the esterification reaction of diols and polyols. For example, in the reaction of methyl glucoside and benzoic acid, the yield of primary alcohol esterification products reached 80% (Figure 5). This strategy offers a novel approach to the selective modification of carbohydrates.
Figure 4: Esterification reaction promoted by HBTU
Figure 5: Selective esterification reaction mediated by TBTU
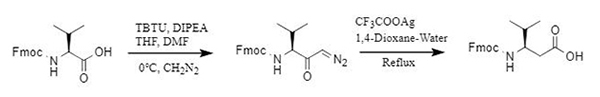
3. TBTU promotes the skeleton reconstruction of α-amino acids to β-amino acids.
The Babu team established a TBTU-Ag+ synthesis system to achieve efficient conversion of α-amino acids to β-amino acids. Under alkaline conditions, TBTU is used to react the amino-protected α-amino acid with diazomethane to obtain a diazoketone intermediate, and then, with the participation of Ag+, a Wolff rearrangement is performed to obtain a β-amino acid with one more carbon. Compared to mixed anhydrides, carbodiimide activation, and other methods, it has the advantages of high yield, fewer by-products, and easy removal.
Figure 6: Transformation from α-amino acid to β-amino acid
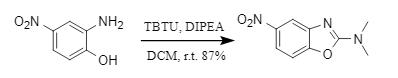
4. TBTU participates in the modular assembly of azole compounds
TBTU breaks through the single functional positioning of traditional condensation reagents and can also directly participate in the construction of heterocycles as a reaction substrate. The Krasavin team developed a method for synthesizing N, N-dimethylazoles, which can form a ring with readily available oximes, amines, and hydrazides under alkaline conditions to obtain the corresponding azole compounds (Figure 7).
Figure 7: HBTU participates in the synthesis of azole compounds
In summary, TBTU not only efficiently drives the formation of structural units, such as amide bonds and ester bonds, as a multifunctional condensation reagent but also participates in the modular assembly of azole compounds as a reaction unit, demonstrating its important application value. After 22 years of relentless efforts and accumulation, Haofan Bio has continued to deepen its roots in the field of global peptide synthesis reagents. It has now developed into a leading company with a wide range of customized product coverage capabilities and significant advantages in large-scale production. It can now supply condensation reagents of various specifications, from the first to the fourth generation, to meet the specific needs of different customers. We sincerely invite customers interested in this product to contact us to learn more about the product details and explore potential cooperation opportunities.
References:
[1] Dumpala, M.; Srinivas, B.; Krishna, PR First Total Synthesis of Jomthonic Acid A1 [J]. Synlett, 2020, 31, 69-72.
[2] Sturabotti, E.; Vetica, F.; Leonelli, F.; et al. N-Acetyl-L-phenylalanine Racemization during TBTU Amidation: An In-Depth Study for the Synthesis of Anti-Inflammatory 2-(N-Acetyl)-L-phenylalanylamido-2-deoxy-D-glucose (NAPA) [J]. Molecules, 2023, 28, 581.
[3] Saeed, B.; Maryam, M.; Rasoul, E. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-Tetramethyluronium Tetrafluoro Borate (TBTU) as an Efficient Coupling Reagent for the Esterification of Carboxylic Acids with Alcohols and Phenols at Room Temperature [J]. Chin. J. Chem., 2008, 26, 6.
[4] Movassagh, B.; Balalaie, S.; Shaygan, P. A new and efficient protocol for the preparation of thiol esters from carboxylic acids and thiols in the presence of 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) [J]. ARKIVOC, 2007, 47-52.
[5] Twibanire, JK; Grindley, TB Efficient and Controllably Selective Preparation of Esters Using Uronium-Based Coupling Agents [J]. Org. Lett., 2011, 13.
[6] Patil, BS; Vasanthakumar, G.; Babu, VVS Synthesis of β-Amino Acids: 2-(1H-Benzotriazol-1 yl)-1,1,3,3-tetramethyluronium Tetrafluoroborate (TBTU) for Activation of Fmoc-/Boc-/Z-α-Amino Acids[J]. Synth. Commun., 2006, 33, 3089-3096.
[7] Lukin, A.; Kalinchenkova, N.; Krasavin, M.; et al. Diversity-oriented synthesis of N, N-dimethylamino-substituted azoles employing TBTU[J]. Tetrahedron Lett., 2018, 59, 2732-2735.