N, N, N', N'-Tetramethylchloroformamidine hexafluorophosphate, referred to as TCFH, is a green and versatile condensation reagent. It is particularly good at condensation reactions between large sterically hindered carboxylic acids and inactive amines, effectively breaking through the limitations of many similar reagents in such reactions. TCFH combined with other reagents can form active intermediates such as acyl chlorides, anhydrides, and acyl imidazoles, thereby achieving efficient construction of amide bonds or ester bonds.
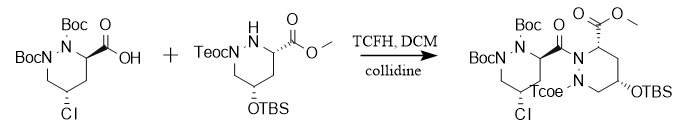
In organic synthesis and drug development, TCFH is usually used as a condensation reagent for the in-situ generation of acyl chlorides. Taking the synthesis of piperazinemycin A as an example, TCFH generates acyl chlorides with carboxylic acids, thereby promoting the coupling between piperazine acids, and demonstrating its potential for application in the synthesis of complex molecules.
TCFH is not only an effective condensation reagent itself but also a precursor of many complex condensation reagents, such as TFFH, HBTU, etc.
More importantly, the combination of TCFH and different bases can easily realize the condensation reaction with large sterically hindered acids and inactive amines as substrates, the synthesis of macrolides, and thioesters.
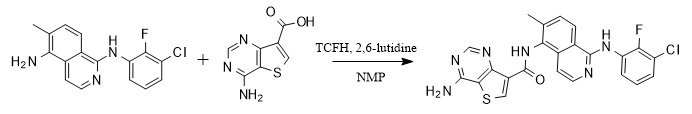
In terms of building amide bonds, TCFH is particularly good at dealing with amine compounds with large steric hindrance or weak nucleophilicity. For example, in the synthesis process of the pan-RAF inhibitor Belvarafenib, TCFH showed excellent performance in the final coupling step and is considered to be the best condensation reagent.
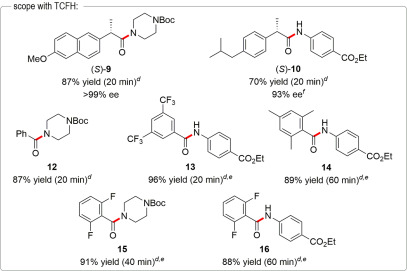
In addition, Gregory L. Beutner's team conducted a relatively in-depth study on the combination of TCFH-NMI, revealing the excellent performance of this combination in acid-amine condensation reactions. For details, please refer to our previous article "The Golden Combination for Efficient Acid-Amine Condensation: TCFH-NMI".
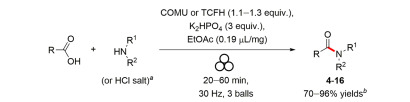
Mechanochemical synthesis is a green synthesis method that is solvent-free, fast in reaction speed easy to separate, and is in line with sustainable development. Riina Aav's team developed a mechanochemical synthesis method based on the TCFH-K2HPO4 and TCFH-NMI system, which can efficiently achieve the construction of amide bonds and has minimal effect on the ortho-stereocenter.
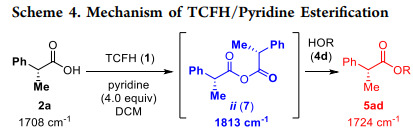
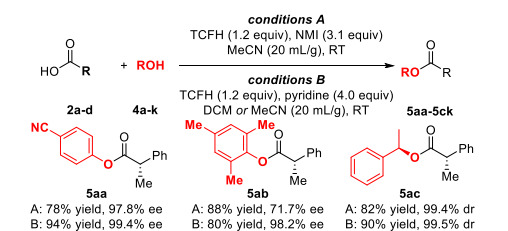
TCFH has demonstrated its wide range of chemical activity and diverse application potential in the field of chemical synthesis. In the esterification reaction, the TCFH-Py system has high reactivity and can efficiently prepare the target ester compounds by forming anhydride active intermediates.
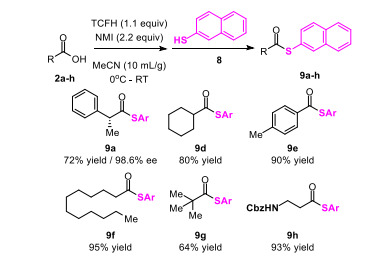
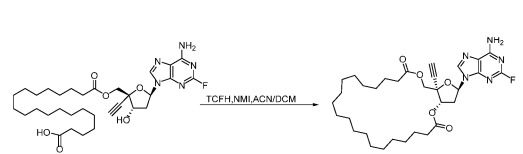
In addition, TCFH can also be used in the synthesis of thioesters and macrolides, further proving its important value in the field of organic synthesis.
Synthesis of thioesters:
Synthesis of macrolides:
In general, TCFH is a widely used condensation reagent with the ability to efficiently synthesize amide and ester compounds. The reagent can be flexibly matched with different additives. By adjusting the reagent ratio and reaction conditions, the best reaction effect can be achieved, and the occurrence of side reactions such as diastereomerization can be reduced, providing more synthetic options for scientific researchers. As the world's leading supplier of peptide synthesis reagents, we are committed to providing customers with high-quality, diverse specifications of TCFH reagents. At the same time, the first to fourth-generation condensation reagents mentioned in the article are all on sale to meet the diverse needs of different customers. Friends with needs are welcome to call us for inquiries.
References:
[1] Kennedy, JP; Lindsley, CW Progress towards the synthesis of piperazimycin A: synthesis of the non-proteogenic amino acids and elaboration into dipeptides[J]. Tetrahedron Lett. 2010,51,2493-2496.
[2] Zell, D.; Dalziel, ME; Gosselin, F.; et al. An Efficient Second-Generation Manufacturing Process for the pan-RAF Inhibitor Belvarafenib[J]. Org. Process Res. Dev. 2021, 25, 2338-2350.
[3] Beutner, GL; Young, IS; Ye, QM; et al. TCFH−NMI: Direct Access to N‑Acyl Imidazoliums for Challenging Amide Bond Formations[J]. Org. Lett. 2018, 20, 4218-4222.
[4] Dalidovich, T.; Mishra, KA; Aav, R.; et al. Mechanochemical Synthesis of Amides with Uronium-Based Coupling Reagents: A Method for Hexa-amidation of Biotin[6]uril[J]. ACS Sustainable Chem. Eng. 2020, 8, 15703-15715.
[5] Luis, NR.; Chung, KK; Vosburg, DA; et al. Beyond Amide Bond Formation: TCFH as a Reagent for Esterification[J]. Org. Lett. 2023.
[6] De La Rosa, MA; Miller, JF; Samano, V.; et al. Compounds useful in HIV therapy. WO 2020178767 A1, 2020.