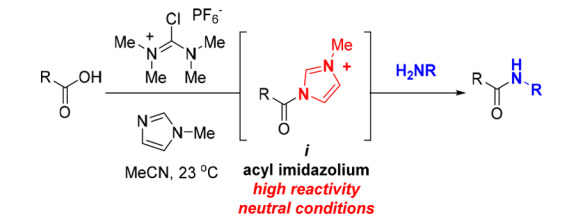
This system appears in the form of a combination of TCFH and NMI (N-methylimidazole), which can generate highly reactive N-acyl imidazolium salts in situ. This golden combination can not only acylate inactive amines (anilines) with high yields but also no diastereoisomerization is found during the reaction. The reaction conditions are mild, and it does not need to be carried out under an inert atmosphere or strictly anhydrous conditions. The by-products produced have good water solubility, which facilitates subsequent product purification. In addition, the system is also suitable for the esterification of alcohols and thiols. The figure below is the general formula of the TCFH-NMI-mediated condensation reaction:
![highfine highfine]()
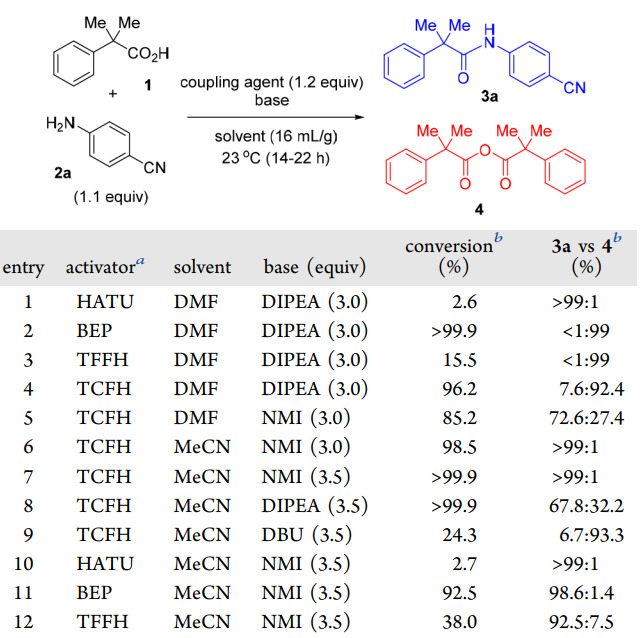
In the research of Gregory L. Beutner's team, they compared other commonly used condensation reagents and bases and found that these condensation reagents had low conversion rates in achieving the conversion of inactive amines, and often could only stay at active esters or form anhydride byproducts. In contrast, the TCFH-NMI system can achieve acid-amine condensation reactions gently and efficiently.
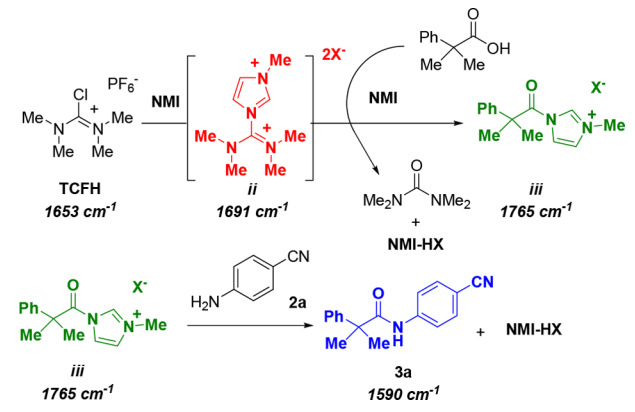
The process of forming amide bonds in the TCFH-NMI system involves two intermediates, as follows:
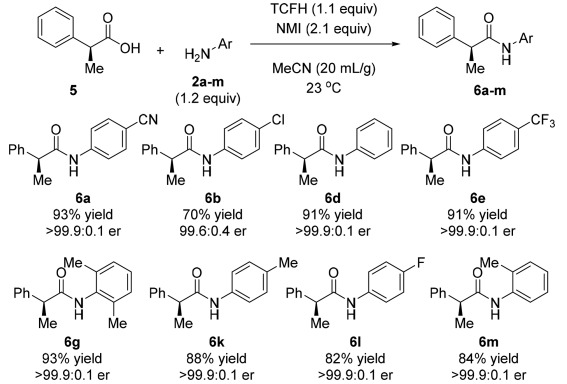
In addition, to broaden the application prospects of the TCFH-NMI system, the team also studied the condensation reaction of acids containing α-stereo centers and inactive amines and compared them with other condensation reagents. The results showed that the TCFH-NMI combination can not only efficiently complete the condensation reaction, but also keep the configuration of the α-stereo center intact, showing a wide range of substrate applicability, while other condensation reagents have low conversion rates, and even if BEP and other reagents have high conversion rates, they cannot maintain stereospecificity.
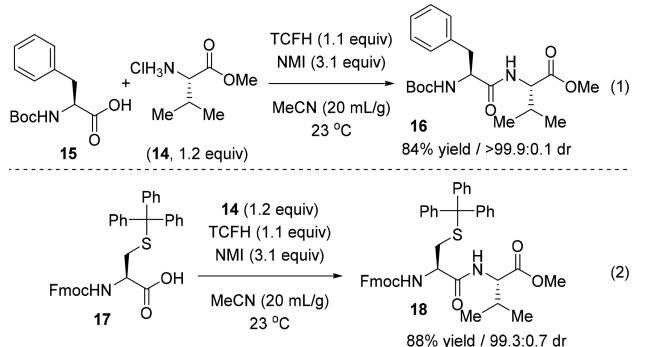
It is worth mentioning that the TCFH-NMI combination also performs well in peptide coupling and can better maintain the integrity of the chiral center.
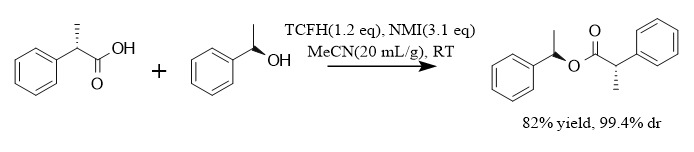
In addition to the synthesis of amide bonds, the TCFH-NMI system can also effectively construct ester bonds, as shown in the figure:
In general, the golden combination of TCFH-NMI, with its excellent performance, can generate acyl imidazolium salts in situ under mild conditions and then efficiently synthesize challenging amide bonds. The mild reaction conditions and good water solubility of the byproducts greatly simplify the subsequent treatment and separation work and provide an effective synthesis method for the reaction of large sterically hindered acids and non-active amines. As a global leading supplier of peptide synthesis reagents, our company can provide high-quality reagents such as TCFH and NMI. At the same time, our first to fourth-generation condensation reagents are all on sale. Friends with needs are welcome to call us for inquiries.
References:
[1] Beutner, GL; Young, IS; Ye, QM; et al. TCFH−NMI: Direct Access to N‑Acyl Imidazoliums for Challenging Amide Bond Formations[J]. Org. Lett. 2018, 20, 4218-4222.
[2] Luis, NR.; Chung, KK; Vosburg, DA; et al. Beyond Amide Bond Formation: TCFH as a Reagent for Esterification[J]. Org. Lett. 2023.