1. Molecular Characteristics of TriAT-tBu:
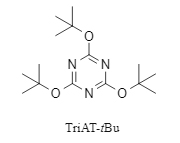
TriAT-tBu (structure shown in Figure 1) is a non-hygroscopic crystalline solid that is non-irritating and non-allergenic. It remains stable even after being exposed to air for up to one year, making it easy to store and handle.
Figure 1. Structure of TriAT-tBu
2. Mechanism of Action of TriAT-tBu:
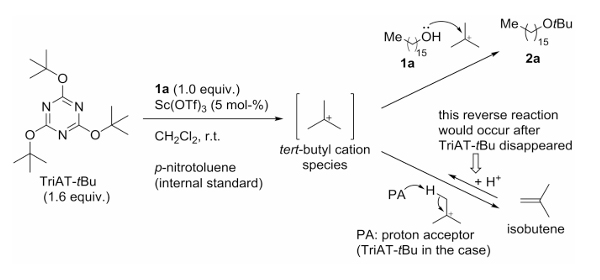
The mechanism of TriAT-tBu is as follows: taking the tert-butylation of alcohols as an example, TriAT-tBu rapidly releases a tert-butyl cation in solution, which then proceeds through two possible pathways to achieve tert-butylation.
Pathway 1: The tert-butyl cation directly reacts with the alcohol to form the corresponding tert-butyl ether, which proceeds rapidly.
Pathway 2: The tert-butyl cation abstracts a proton from a proton acceptor in the solution to generate isobutylene, which then reacts with the alcohol to yield the tert-butyl ether at a slower rate.
Kunishima’s team continuously monitored the reaction process. During the stage when TriAT-tBu was still present, Pathway 1 was the dominant and faster route for product formation. After TriAT-tBu was consumed, Pathway 2 became the main route, relying on isobutylene to regenerate the tert-butyl cation, but with significantly lower efficiency. Therefore, to suppress isobutylene formation during use, TriAT-tBu is best added slowly as a solution to maintain a low reaction concentration and improve efficiency.
Figure 2. Mechanism of TriAT-tBu
3. Functions of TriAT-tBu:
In the presence of an acid catalyst, TriAT-tBu releases tert-butyl cations that enable efficient tert-butyl protection of alcohols and carboxylic acids.
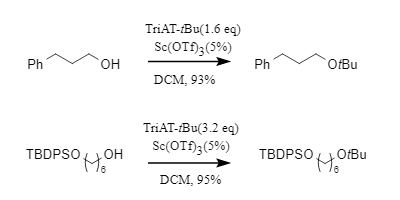
1. Formation of tert-butyl ethers with alcoholsWhen reacting with alcohols, the tert-butylation is performed in dichloromethane as solvent and in the presence of an acid catalyst such as triflic acid or scandium triflate. Ether solvents (e.g., tetrahydrofuran) are not effective reaction media. TriAT-tBu shows high reactivity toward primary and secondary alcohols, is compatible with various functional groups such as TBDPS and Boc (only when using scandium triflate as catalyst), and preserves the stereochemistry of chiral compounds. However, tertiary alcohols generally do not react with TriAT-tBu to form tert-butyl ethers.
Figure 3. tert-Butylation of alcohols by TriAT-tBu
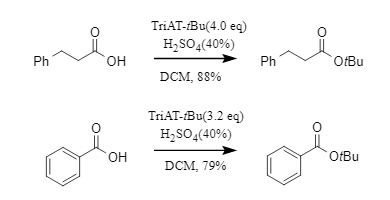
2. Formation of tert-butyl esters with carboxylic acidsWhen reacting with carboxylic acids, scandium triflate gives tert-butyl esters in moderate yields, while sulfuric acid promotes the reaction more efficiently. Both aliphatic and aromatic acids can be smoothly converted into their corresponding tert-butyl esters, demonstrating broad substrate applicability.
Figure 4. tert-Butylation of carboxylic acids by TriAT-tBu
In summary, the novel tert-butylating reagent TriAT-tBu offers high stability, non-hygroscopicity, and ease of handling. Under acid-catalyzed conditions, it enables tert-butyl protection of alcohols and carboxylic acids in moderate to excellent yields. Its development not only addresses the limitations of traditional reagents but also provides a new approach for introducing tert-butyl groups in synthetic chemistry, with significant research and application value.
After 22 years of continuous effort and experience, Haofan Biotech has become a leading enterprise in the global peptide synthesis reagent industry, with extensive capabilities in customized product development and large-scale production. We sincerely invite interested customers to contact us for further product details and potential collaboration opportunities.
References:
[1] Yamada, K.; Fujita, H.; Kunishima, M., et al. Development of a Triazine-Based tert-Butylating Reagent, TriAT-tBu. Eur. J. Org. Chem. 2016, 23, 4093–4098.