10/8/2021
Trimethylsilylethoxycarbonyl (Teoc) differs from Cbz, Boc, Fmoc, and Alloc. It is very stable to acids, most alkalis, and noble metal catalysis. In its presence, Cbz, Boc, Fmoc, Alloc, etc. It can be selectively deprotected; its removal is usually carried out in the fluoride anion.
Application of Trimethylsilylethoxycarbonyl (Teoc) Protecting Group
Structural formula:

The introduction mechanism of trimethylsilylethoxycarbonyl (Teoc):
The introduction of the Teoc protecting group takes Teoc-Osu as an example. The mechanism is as follows:

The removal mechanism of trimethylsilylethoxycarbonyl (Teoc):

Application of Trimethylsilylethoxycarbonyl (Teoc):
In recent years, the use of silicon-based reagents for protecting active functional groups has been developing rapidly, and trimethylsilylethoxycarbonyl (Teoc) is the most used as a protecting agent for amino groups in organic synthesis and biochemistry. Trimethylsilylethoxycarbonyl (Teoc) is different from Cbz, Boc, Fmoc, and Alloc. It is very stable to acids, most alkalis, and noble metal catalysis. In its presence, Cbz, Boc, Fmoc, and Alloc, etc. It can be selectively deprotected, and its removal is usually carried out in fluoride anions, such as TBAF, TEAF, and HF. Alternatively, TFA can also selectively deprotect the trimethylsilylethoxycarbonyl group.
Application 1: Protection of conventional amino groups
The Teoc protecting group usually uses Teoc-Cl, Teoc-NT, Teoc-OSu, or Teoc-OBt to react with amino compounds in the presence of a base. The base can be organic, pyridine, triethylamine, or an inorganic base, sodium bicarbonate so that Teoc-protected amino derivatives can be obtained.
In the study of carbamate-protected amino groups, Mamoru explained that the reaction stability of the Teoc-protecting group is high; the protecting reagent does not easily absorb moisture. It can even be exposed to the reaction, and the nitroimidazole produced using Teoc-NT can also be recycled. Sometimes, high-purity Teoc reagent can obtain amino derivatives with higher purity without tedious column chromatography purification.
Example 1: Teoc-OBt/triethylamine system yield 92%

Example 2: Teoc-Cl/sodium bicarbonate system, yield 87%

Example 3: Teoc-Osu/triethylamine system yields 95%

Example 4: Teoc-NT/triethylamine system yield 98%

Application 2: Amino protection of nucleoside derivatives
Since the introduction of the Teoc protecting group is relatively clean and easy to handle, it is also often used in nucleotide protection. For example, cytidine derivatives can introduce Teoc groups without a base, as shown in the figure below:

Similarly, the 6-NH2 of adenosine derivative 14A is also very successful in selectively introducing Teoc groups under neutral conditions. Still, the reaction of the amino group of guanosine derivatives is difficult to carry out without a base, and triethylamine is needed. As the base, Teoc-Cl is the introduction agent, as shown in the figure below:

Although the Teoc group has been widely used in nucleoside derivatives, the reports indicate that the introduction of the Teoc group to the 2-NH2 of 2,2'-deoxyguanosine derivatives has not been successful.
Application 3: Amino protection of amino acid derivatives
Due to the diversity of amino acid derivatives, protecting their amino groups is also more important. Various protecting groups are selected according to different needs, and the Teoc protecting group is also commonly selected.
Richard's experimental table on Teoc protection of amino acid derivatives:
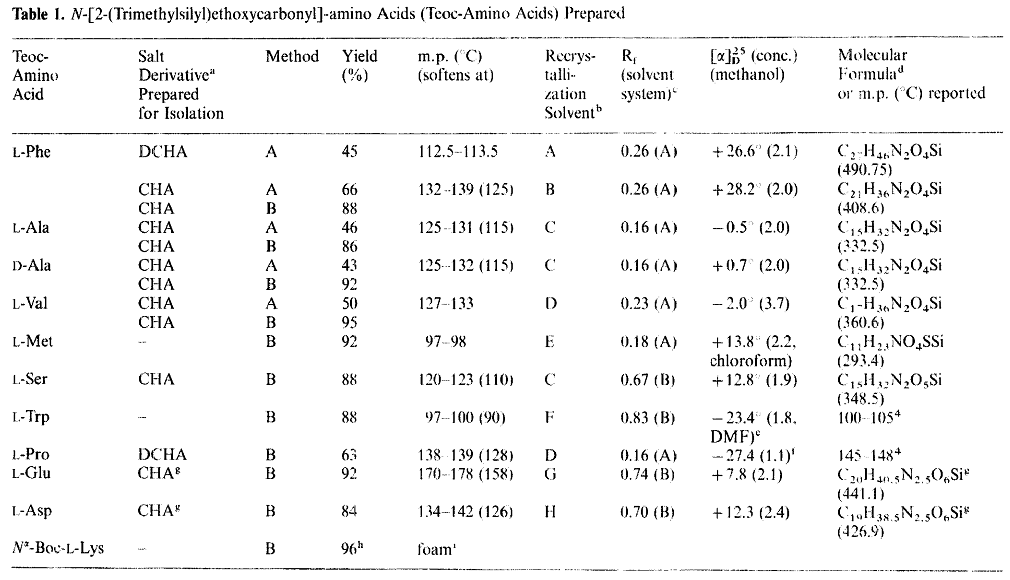
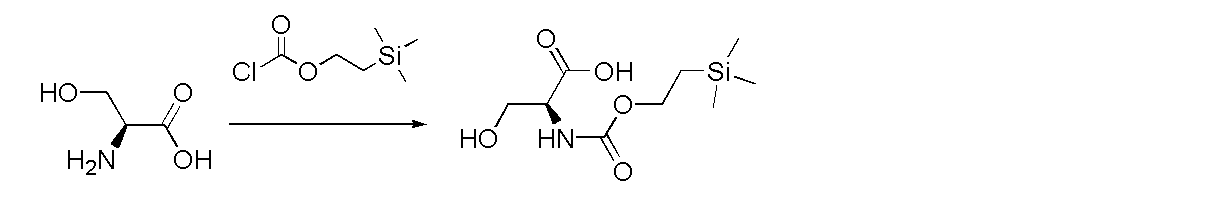
L-serine uses triethylamine as the base and Teoc-Cl as the introduction agent, as shown in the figure below:
Teoc protection of L-glutamic acid is as follows:

It can be seen from the above reports that the Teoc protecting group has strong applicability in amino acid protection.
Experimental operation:
Protection on instance 1:
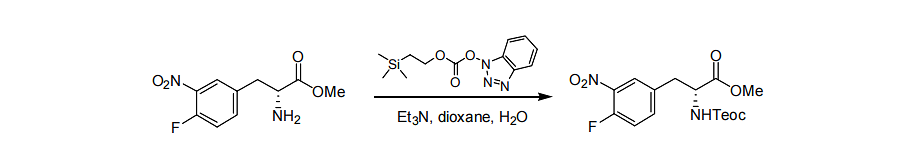
The starting material (3.0 g, 12.4 mmol, 1.0) was added to dichloromethane (50 mL), followed by triethylamine (3.25 g, 32.2 mmol, 2.6). Then, Teoc-OBt (3.52 g, 13.63 mmol, 1.1), control the temperature at 20-25°C until the raw materials are completely consumed. Add 20mL of saturated potassium hydrogensulfate solution to the reaction, separate the organic phase and wash it with 20mL of saturated brine, dry the organic phase with anhydrous magnesium sulfate, filter, and concentrate to obtain 4.4g of the product with a yield of 92%.
Example 2 deprotection:

The raw material (5.5g, 18.68mmol, 1.0) was added to tetrahydrofuran (70), and tetrabutylammonium fluoride (7.33g, 28.02mmol, 1.5) was added in batches and reacted at room temperature until the raw material was completely consumed. The reaction solution was concentrated to remove tetrahydrofuran, and the crude product was obtained by column chromatography, with a yield of 85%.
References:
[1]Boger, Dale L; Kim, Seng Heon et al., J. Am. Chem. Soc., 2001, 123(9), 1862-1871;
[2]Shute, Richard; Rich, Daniel H; Synthesis, 1987, 4, 346-349
[3] Mamoru Shimizu; Mikiko Sodeoka., ORGANIC LETTERS., 2007 , Vol. 9, No. 25, 5231-5234
[4]Seng Heon et al., J. Am. Chem. Soc., 2000, 122(30), 7416-7417
[5]Gugiu, Bogdan G; Salomon, Robert G; Org. Lett., 2003, 5(16), 2797-2800
[6]Tius, Marcus A; Thurkauf, Andrew; Tetrahedron Lett., 1986, 27(38), 4541-4544
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.