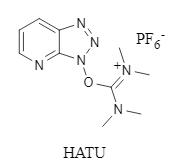
Figure 1: HATU structure
1. HATU-mediated amide bonding reaction
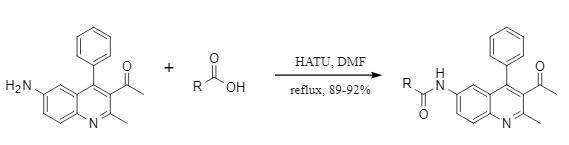
HATU is an efficient condensation reagent with a mechanism of action similar to that of TBTU and HBTU. It forms carboxylic acid active esters, and then obtains amide compounds under the attack of nucleophilic reagents (Figure 2). It is especially suitable for substrates with large steric hindrance and can effectively inhibit the racemization of chiral centers. In the synthesis research of acidophilin AC, the Behera team systematically investigated the effects of condensation reagents such as PyBOP, T3P, and DCC on the coupling of key fragment tripeptides, and found that only HATU can obtain products with high stereoselectivity (Figure 3), which fully demonstrated its technical advantages in the synthesis of complex peptides.
Figure 2: HATU-mediated amide bonding reaction
Figure 3: HATU-mediated synthesis of chiral tripeptides
2. HATU-promoted one-pot synthesis strategy for preparing diamides
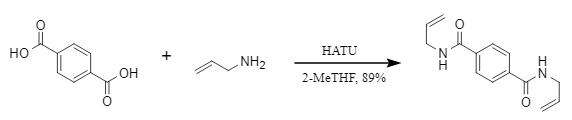
Pednekar's team innovatively developed a one-pot synthesis strategy for forming diamides using HATU-driven dicarboxylic acids and amine compounds under mild conditions, successfully achieving the construction of diamides. This method has high substrate universality and is suitable for a wide range of dibasic aromatic and aliphatic carboxylic acids. The synthesis yield is above 70%, offering a new approach to the synthesis of polyamides.
Figure 4: HATU-driven one-pot synthesis of diamide
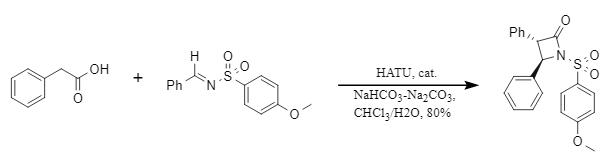
3. HATU promotes the construction of cyclic lactams
The Magriotis team established a new HATU-mediated β-lactam stereoselective synthesis strategy. The system forms an active ester with the carboxylic acid through HATU. It then forms an enolate anion under the action of a chiral isothiourea catalyst and a base. Finally, it reacts with an imine to form a β-lactam with an enantiomeric excess (ee%) value of 96%. The efficient β-lactam synthesis method provides a new idea for subsequent drug synthesis and the construction of multifunctional chiral molecules.
Figure 5: HATU promotes the construction of the lactam skeleton
4. HATU promotes the synthesis of aryl benzimidazoles
At the common benzimidazole structural units in drug molecules, the Ameta team has developed a strategy for the efficient one-pot synthesis of N-methylbenzimidazole derivatives via the HATU/HOBt system (Figure 7). This method uses HATU and HOBt to construct the N-methylbenzimidazole skeleton from N-methyl o-phenylenediamine and various aromatic carboxylic acids. It is worth noting that amine substrates such as heteroaromatic amines and other N-alkyl-substituted o-phenylenediamines are limited, and the corresponding benzimidazole derivatives cannot be obtained.
Figure 6: HATU-mediated synthesis of arylbenzimidazoles
In summary,
HATU is a powerful peptide synthesis reagent with advantages including high efficiency and excellent stereoselectivity, and it plays a crucial role in both peptide synthesis and medicinal chemistry. After 22 years of relentless efforts and accumulation, Suzhou Highfine Bio has continued to deepen its presence in the global peptide synthesis reagent market. It has now developed into a leading company with extensive customized product coverage capabilities and significant advantages in large-scale production. It can now supply first- to fourth-generation condensation reagents with diversified specifications to meet the specific needs of various customers. We sincerely invite customers interested in this product to contact us to learn more about the product details and explore potential cooperation opportunities.
References:
[1] Murray, J.; Nicolette, J.; Neft, R.E.; Peptide Bond-Forming Reagents HOAt and HATU are
not Mutagenic in the Bacterial Reverse Mutation Test[J]. Environ. Mol. Mutagen., 2016, 57, 236-240.
[2] Chawla, PA; Shome, A.; Jha, KT Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium (HATU): A Unique Cross-Coupling Reagent [J]. SynOpen, 2023, 7, 566-569.
[3] Mallesham, P.; Raghavulu, K.; Behera, M.; et al. Synthesis of Acidiphilamide A–C: Secondary Metabolites from the Genus Streptacidiphilus[J]. SynOpen, 2023, 7, 130-139.
[4] Bhatt, V.; Samant, SD; Pednekar, S. Efficient One-pot HATU Mediated Coupling of Dicarboxylic Acid and Amines for the Synthesis of Diamide at Ambient Temperature[J]. Lett. Org. Chem., 2017, 14, 764-768.
[5] Demos, V.; Karakoula, A.; Magriotis, PA; et al. Catalytic enantioselective Gilman-Speeter synthesis of β-lactams by nucleophilic activation of carboxylic acids[J]. Results in Chemistry, 2023, 6.
[6] Duan, YT; Parmar, TH; Ameta, RK; et al. 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo [4,5-b]pyridinium 3-Oxide Hexafluorophosphate (HATU)/Hydroxybenzotriazole (HOBT)-based One-Pot Cyclization of N-Substituted 2-Arylbenzimidazole Derivatives [J]. Russ. J. Org. Chem., 2020, 56, 856-862.